PhysioMimix® OOC Microphysiological Systems
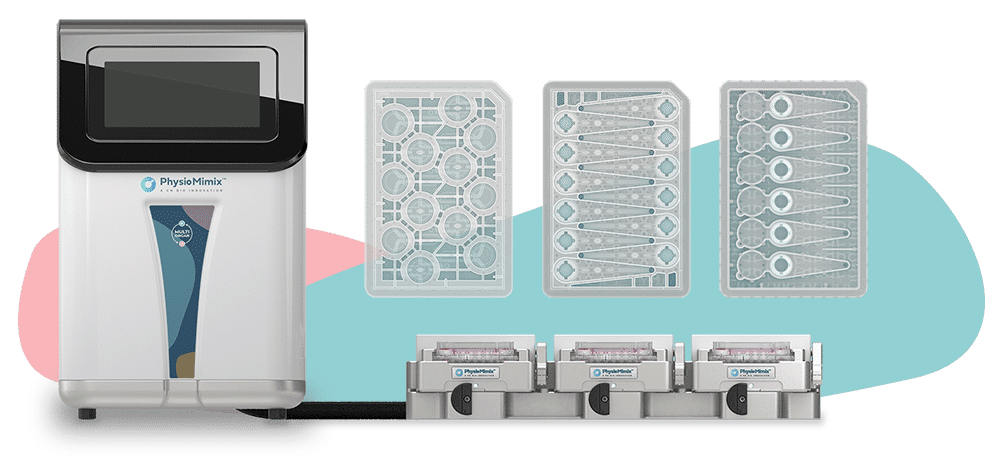
Transforming the way human-relevant preclinical data is generated
Take a tour with our animated videos

PhysioMimix®
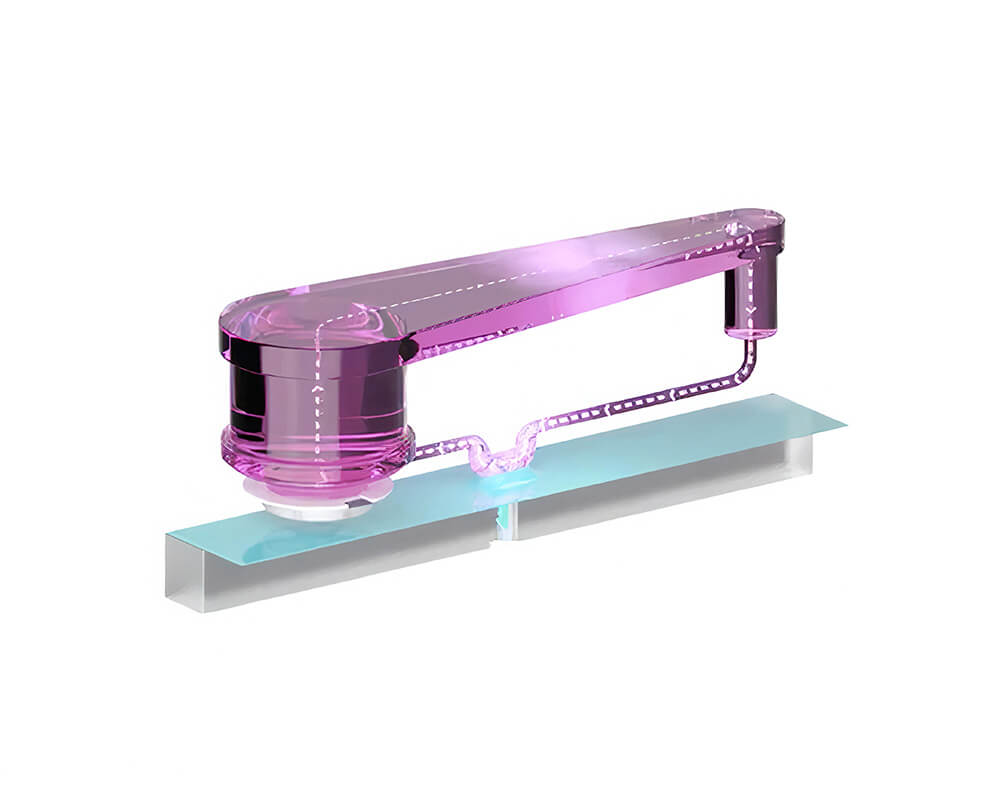
Single-organ system
PhysioMimix®
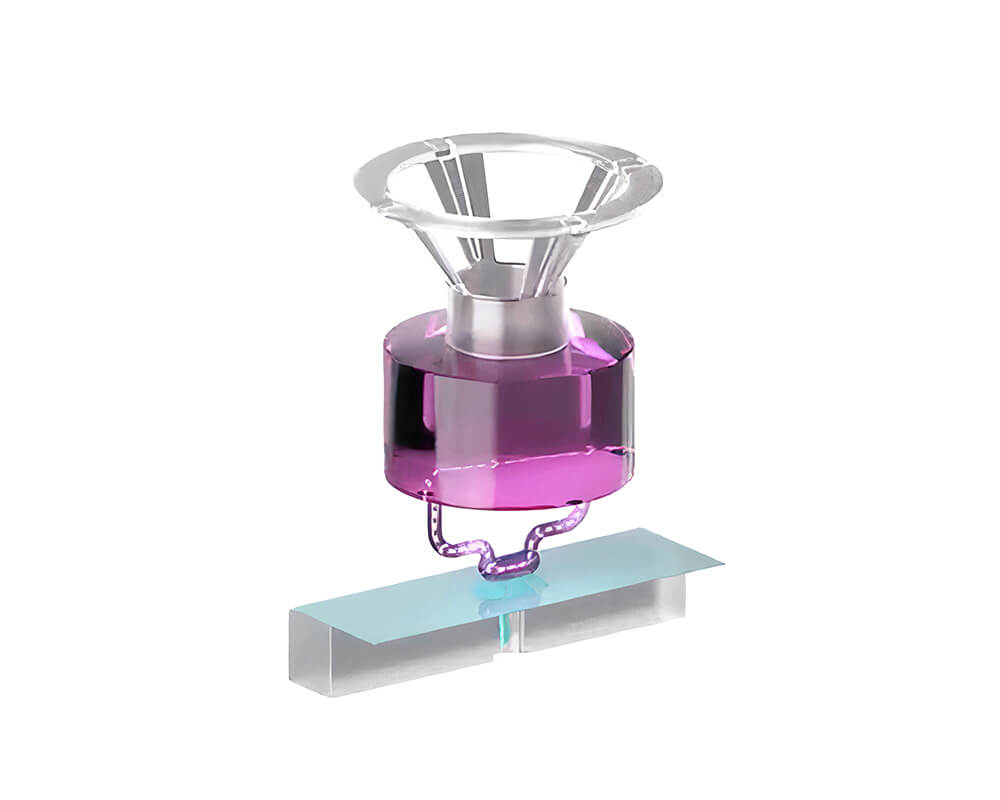
Multi-organ system
PhysioMimix Single-organ Animated Video
PhysioMimix Multi-organ Animated Video
Generate human-specific pre-clinical safety and efficacy data with pioneering PhysioMimix single- and multi-organ-on-a-chip solutions.
PhysioMimix organ-on-a-chip (OOC) systems enable you to culture microtissues that mimic the structure and function of human tissues and organs in vitro.
Our perfused systems provide accurate fluidic flow to replicate the bloodstream, promoting 3D organ model formation and supporting the inclusion of circulating immune cells to mimic the immune system.
Predictive human organ models can be used to identify drug targets, understand drug ADME properties, mechanisms of efficacy and toxicity to more closely predict clinical outcomes.
By improving the in vivo predictivity of in vitro data, PhysioMimix OOC systems help companies like yours to develop safe and efficacious therapeutics, faster and more cost-effectively, while reducing your dependence on animal usage.
How translatable is your data?
Human-relevant data generated by the PhysioMimix OOC complements that derived using entrenched 2D cell culture and animal studies to support more confident decision-making across many drug discovery stages from target discovery right through to preclinical development.
Limitations of current techniques
- In vitro 2D cell culture models lack physiological relevance
- In vivo animal models lack human relevance; studies are slow, expensive, and ethically undesirable
- Inter-species differences limit the suitability of animals for testing human-specific drug modalities
Advancements with PhysioMimix
- Faithfully recapitulate the phenotype and function of human organs and tissues in vitro
- Addresses the relevance limitations of standard preclinical models to validate or query their results
- Provides a rapid, usable, cost-effective and more human-relevant alternative
What’s required to accurately model and measure human physiology?
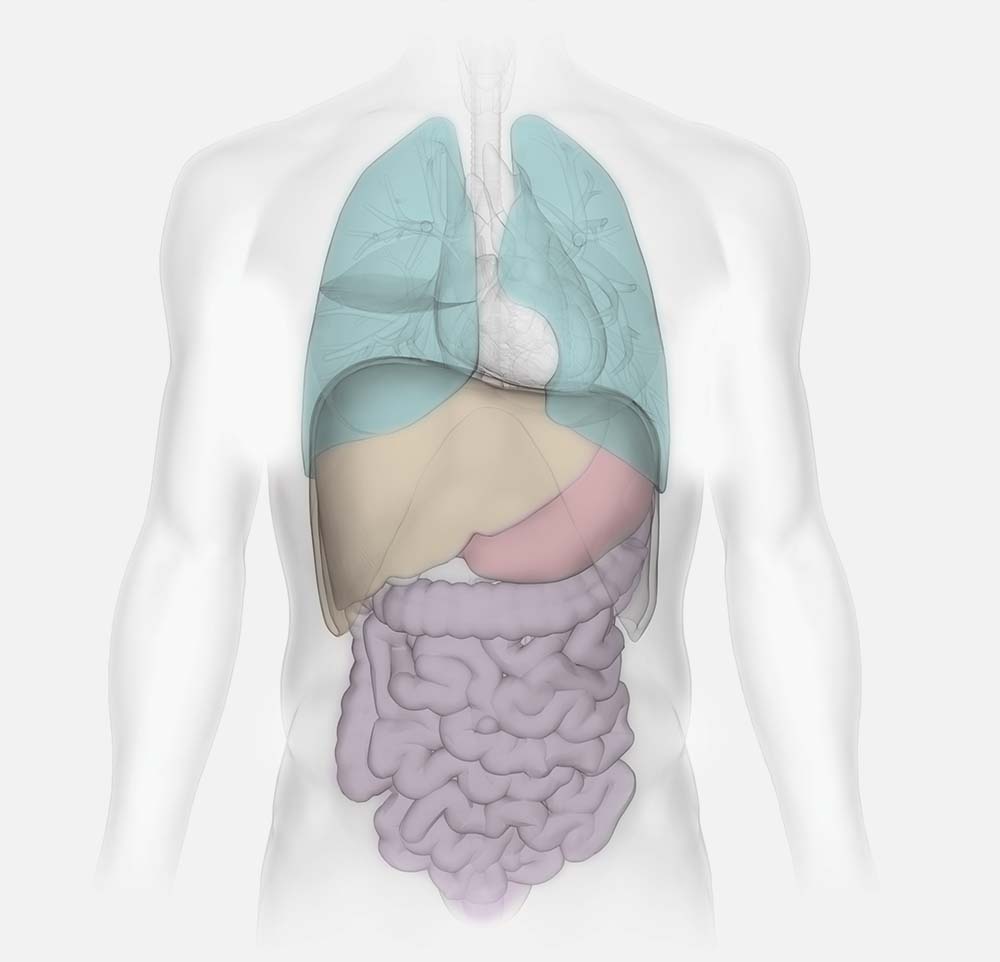
PhysioMimix OOC enables:
- the co-culturing of human cells to faithfully recreate human organs and tissues
- perfused scaffolds to promote 3D organ model formation
- compatibility with inserts for apical and basolateral tissue growth to recreate a biological barrier
- recirculating fluidic flow delivering biomechanical stimulus, oxygen, and nutrients
- adjustable inter- and intra-organ flow rates to enhance physiological relevance
- viability, function, and phenotype to be maintained over many weeks to enable longer-term chronic disease and dosing studies
- deep mechanistic insights via large sampling volume (up to 1 mL) and individual microtissue analysis
- modeling of the immune system via the inclusion of circulating and tissue-specific immune cells
Preclinical toolbox comparison
How does OOC compare to conventional preclinical approaches?
| In vitro 2D cell culture | In vitro 3D spheroid | In vivo animal models | Organ-on-a-chip | |
| Human relevance | ||||
| Complex 3D organs and tissues | ||||
| (Blood)/Flow perfusion | ||||
| Innate & adaptive immune system | ||||
| Longevity | < 7 days | < 7 days | > 4 weeks | ~ 4 weeks |
| Acute and chronic dosing | ||||
| New drug modality compatibility | LOW | MEDIUM | LOW | MEDIUM / HIGH |
| Throughput | ||||
| Time to result | FAST | FAST | SLOW | FAST |
| High content data |
Which of our microphysiological systems meets your needs?

PhysioMimix Single-organ Standard System
Entry-level system that enables the creation of in vitro 3D single-organ models to provide deep insights into drug, or disease mechanism of action.
Enables insightful decision making by delivering clinically translatable human data.
Generate Liver-on-a-chip models that mimic the human liver microarchitecture using the PhysioMimix Liver-12 plate.
Generate barrier models (such as Gut- or Lung-on-a-chip) on Transwell® inserts using the PhysioMimix Barrier plate.

PhysioMimix Single-organ HT System

Next-generation system that additionally provides:
6x higher throughput * for comparative studies evaluating drug safety, efficacy and potency.
8x reduction in cost/sample* to reduce adoption barriers.
Enhanced data robustness and reproducibility capabilities for data confidence.
Delivers clinically translatable human data earlier in discovery than ever before.
Compatible with the NEW PhysioMimix Liver-48 plate, plus those of the Single-organ Standard System.
*versus a single-chip alternative

PhysioMimix Multi-organ Standard System

Providing additional functionality over the Single-organ Standard System, the multi-organ variant enables you to interconnect our liver model with an additional organ, such as the gut or lung,
By mimicking how organs interact and communicate as part of a complex system, you benefit from a deeper human-specific mechanistic understanding of disease states and drug behavior.
Comptible with the PhysioMimix Dual-organ plate, plus those of the Single-organ Standard System
PhysioMimix OOC range by task
| Single-organ Systems | Multi-organ System | ||
| Standard | HT | Standard | |
| Mechanistic insights | |||
| Safety, efficacy & potency | |||
| Inter-organ crosstalk | |||
Bringing the benefits of Organ-on-a-chip to more of drug discovery
Customer feedback
The CN Bio MPS system allows hepatic cultures to be grown for weeks with low signs of cytotoxicity or hepatocytes de-differentiation detectable; CN Bio has developed an efficient proprietary protocol to model NASH that shows really high homology with murine models of NASH in terms of transcriptome, inflammatory profiling and pathophysiological events crucial for disease progression
The hepatic co-cultures of hepatocytes, Kupffer and stellate cells that I have tested also behave as expected in vivo with regards to intracellular signalling pathways (such as TGFβ signalling) thus allowing the modeling of chronic liver disease pathophysiology.
I think that the PhysioMimix OOC system will become a “must-have” piece of equipment for labs focussing on liver pathophysiology and tissue-to-tissue interactions.
Michele Vacca, MD PhD
Clinician Research Associate at the Institute of Metabolic Science, University of Cambridge
View more PhysioMimix OOC reviews on SelectScience

Application areas
Disease modeling
Our models functionally mimic the organ and give a realistic expression of disease phenotypes for improved efficacy assessments
Safety toxicology
By closely mimicking in vivo function, our models better predict drug safety to support and accelerate drug development.
ADME
Single- and multi-organ models closely predict human in vivo pharmacokinetics for informed insights into the body’s effect on drugs.
Why choose PhysioMimix?

Lab benchtop ready
Compact and compatible with existing equipment
User friendly
Program and start a run in less than 1 minute
Open-well design
Supports your 2D to 3D cell culture transition

Real-time monitoring
Remove samples for analysis, experiments continue to run

“Set-and-Run” Perfusion
Long-term automated experiments with minimal user input
Tissues and cells
Compatible with a range of pre-formed tissues and cell types for ultimate flexibility
Multi-organ compatible
Connect two organs via microfluidics to study crosstalk

Reduced cost/chip
More experiments, same cost reduces adoption barriers
Data confidence
In-plate replicates, controls and dose-responses
Higher throughput
Generate OOC insights earlier than ever before
PhysioMimix tech spec
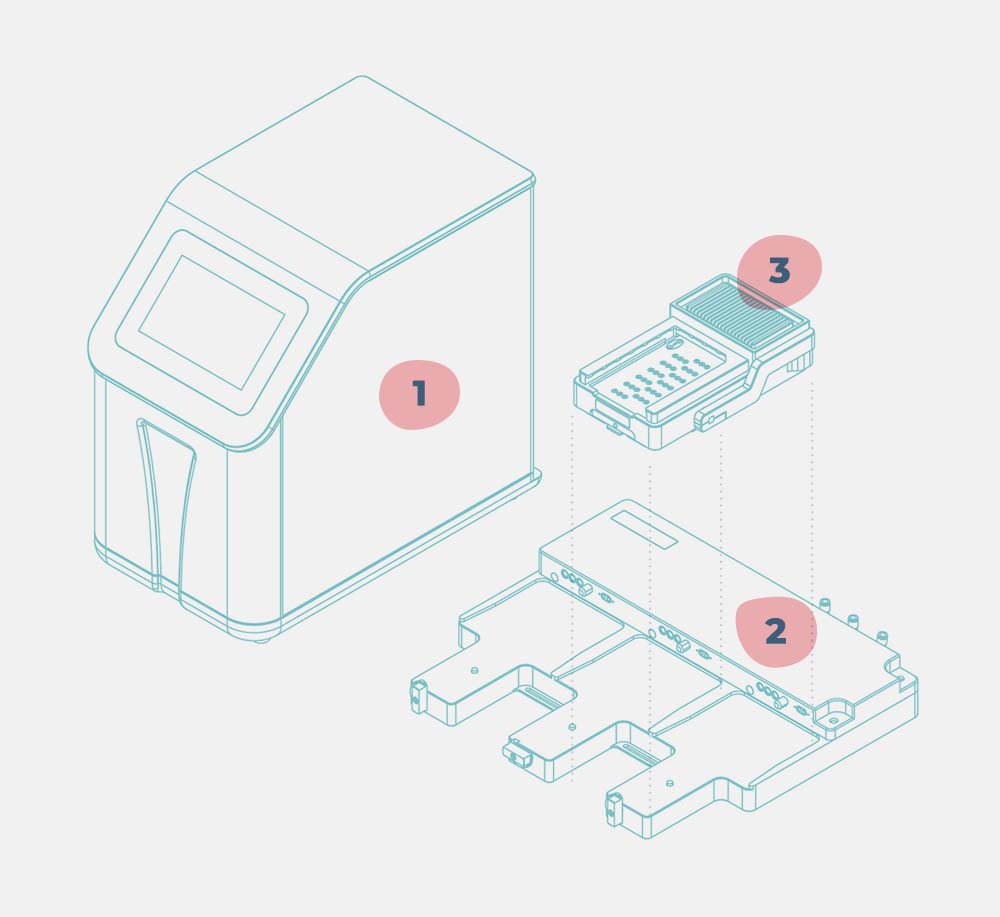
PhysioMimix® OOC systems include the following hardware:
- Controller
- Docking station(s)
- Driver(s)
One standard software licence*
One-year manufacturer’s warranty
* Additional modules are required for certain consumables and applications.
View detailed technical specification
| Product | Dimensions | Mass | Requirements | Cat. No |
|---|---|---|---|---|
| PhysioMimix Controller Controller capable of parallel operation of up to 6 Multi-chip plates mounted on 2 Docking stations | 230 (W) x 430 (D) x 415 (H) mm | 17.5 kg | Power Supply 100-240V~ 50/60Hz Maximum Power Consumption 500W | Single-organ Standard PMX-T1-CON Single-organ HT PMX-T1- HT-CON Multi-organ PMX-M1-CON |
PhysioMimix Docking Station Docking station acts as an interface between the PhysioMimix MPS Driver and Controller | 435 (W) x 380 (D) x 65 (H) mm | 4.4 kg | Incubator with side/rear port. One Docking Station per shelf in a standard cell culture incubator | Single-organ PMX-T1-DS3 Multi-organ PMX-M1-DS3 |
| PhysioMimix MPS Driver One Multi-chip plate per MPS Driver | 135 (W) x 230 (D) x 55 (H) mm | 1.9 kg | Single-organ Standard PMX-T1-MD6 Single-organ HT PMX-T1-MD7 Multi-organ PMX-M1-MD5 |
Frequently asked questions
Note that the terms organ-on-a-chip (OOC) and microphysiological system (MPS) are used synonymously throughout.
How does PhysioMimix® OOC compare to alternative technologies?
A wide variety of Organ-on-a-chip (OOC) systems are available. OOCs vary in shape and format, from the more “simplistic” gravity-driven platforms, providing basic perfusion, through to complex microfluidics systems, that more accurately mimic the bloodstream, such as our PhysioMimix® OOC range of microphysiological systems (MPS). Each has pros and cons and different contexts of use (COU), so different OOC technologies may be used together to improve the overall human relevance of your workflows. To help you to establish which OOC technology is right for your needs, look at our “Top tips for integrating OOC technology into your workflows” infographic.
When comparing to chip-based technology with more physiologically relevant microfluidics, the main advantages of our PhysioMimix OOC Systems are described below:
Ease of use
Despite the complex biology that it mimics, PhysioMimix OOC Systems are simple to install and integrate into existing workflows. Engineers are not required to install the platform; it can be set up in less than an hour by the user. The touch screen interface on PhysioMimix Controller units is very user-friendly. Simply select the right program and press play, there is no need to fine-tune anything but the flow rate. We purposefully chose a plate-based design for consumables to decrease adoption barriers. Customers find the open nature of our OOC system an advantage. For example, when inducing/studying the mechanism of disease or performing temporal studies, it is easy to access the cultures to change experimental conditions, manipulate the models, or remove samples.
To further decrease adoption barriers, we also provide 3D validated cells and consumable plates with pre-coated scaffolds. For customers wishing to recreate our Non-alcoholic Steatohepatitis (NASH) disease model we provide everything required in a kit format (NASH-in-a-box) plus guided software to walk users through the protocol in a step-by-step manner.
Large scale microtissues and media volumes
With up to 1 mL of media available/sample, and microtissue that can easily be recovered, our PhysioMimix OOC Systems enable deeper mechanistic insights to be derived/sample compared to alternative micro-scale chip-based systems. The open-well set up of Multi-chip plates allows for regular sampling of up to 1 ml of media which can be analyzed to identify metabolized and secreted analytes over time, including clinical biomarkers (such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST) for the liver).
The relatively large scale recoverable microtissue allows for microscopy and -omics analysis (particularly next generation sequencing, single cell sequencing, proteomics, lipidomics etc.), to be run in parallel on the same tissue.
Please visit individual application pages for a list of commonly derived endpoint measurements.
High assay sensitivity
The combination of physiologically relevant flow perfusion and the relatively large, human relevant microtissues generated by PhysioMimix OOC Systems, provides high assay sensitivity as explored in more detail in this blog
Single and multi-organ capabilities
The PhysioMimix Multi-organ System provides both single organ and multi-organ capabilities, the latter of which enables bioavailability determination, complex investigational toxicology studies and the discovery of insights that can only be derived by interconnecting organs and tissues together into systems
Throughput and cost/sample
Although not truly high throughput, our systems offer the highest throughput capabilities in class. Our multi-chip plate-based approach has the added benefit of being scalable compared to most OOC solutions where you can only run one experiment, or condition per chip. To increase throughput, we simply need to incorporate more chips into the plate and run more plates simultaneously -rather than redesigning the whole system. One PhysioMimix controller can run up to six Multi-chip plates. Our plates have between six and 48 chips, or wells/plate. This approach also benefits from a reduced cost/sample.
Low non-specific binding consumables
Most chip-based technologies use polymethylsiloxane (PDMS) to create their chip and microfluidics. However, drugs bind highly and non-specifically to PDMS, which is problematic when assessing a compound’s dose dependent effects. When developing our PhysioMimix Multi-chip plates, we choose to use cyclic olefin copolymer (COC), because of its low non-specific binding properties to avoid these concerns (Tsamandouras et al., 2017).
Recapitulating the immune system
An advantage of our system is the possibility to add in both innate, or tissue-specific immune cells and circulating immune cells to assess immune responses. Most chip-based OOC technologies have a flushing-type of microfluidics in which the liquid is flushed from one side of the chip to the other and unable to recirculate through, or around, the cellular tissue. This alternative approach is more limiting for those wishing to investigate immune-mediated responses.
Close collaborative links to the US FDA
We have a long-standing collaboration with multiple research groups within the U.S. Food and Drug Administration (FDA) which led to a co-publication comparing our liver-on-a-chip (or liver MPS)) model to the gold standard of in vitro liver cell culture (Rubiano et al, 2021). This first ever co-publication between a regulator and an OOC developer showcases the great potential of OOC technologies for improving the predictivity of preclinical drug development.
If you’d like to watch a short 15-minute presentation that provides a direct comparison of traditional approaches to genotoxicity testing and commercially available OOC technologies from collaborators at Charles River laboratories click here:
What is the throughput capacity of PhysioMimix OOC Systems?
Firstly, when we talk about throughput, there is generally an inverse relationship between throughput and physiological relevance. High throughput in vitro screens are generally performed using simplistic but convenient 2D immortalized cell-based assays. They usually provide a low number of output measurements from which yes/no decisions can be made at scale, however their ability to predict human outcomes is relatively low. OOC assays provide the opposite as explored in more detail in this blog. In short, OOC assays are more suited to early-stage target identification/validation, lead optimization and late-stage preclinical drug evaluation phases of the discovery and development pipeline. Here, fewer compounds are screened in complex human-relevant preclinical assays that use primary cells. These assays deliver greater sensitivity, higher-content data to provide deeper mechanistic insights and improved data translatability into the clinic.
Our PhysioMimix systems currently offer medium throughput. Each Controller unit system can run up to six Multi-chip plates simultaneously, with six to 48 wells provided within each Multi-chip plate. The number of wells/chips per plate depends on the plate type. For example, our Dual-organ plate contains six wells/chips per plate, our Barrier plate contains 12, and we have two types of Liver plates containing either 12, or 48 chips per plate. Note: to use Liver-48 plates, the PhysioMimix Higher throughput (HT) System is required. The PhysioMimix Multi-organ system is required to run Dual-organ plates.
Does the new PhysioMimix HT System and Liver-48 plate enable you to perform dose-response curves within the same plate?
Absolutely, expanding the number of microtissues from 12 to 48 per plate using the PhysioMimix Single-organ HT System and its Multi-chip Liver-48 plate enables you to run multiple dose-responses within a single plate. You can run two dose-response curves (seven concentration points/curve) on a single plate, or more dose-response curves with fewer points, plus controls, in triplicate. One thing we are excited about is that you can get more consistent data because everything is housed within a single plate, so you don’t have to worry about inherent variability between plates or chips.
The additional advantage of the Liver-48 plate is that everything (cells, media, reagents) is ~75% smaller so this also reduces your cost/condition.
What are the main components of PhysioMimix OOC Systems?
Our PhysioMimix® OOC Systems are comprised of a controller unit that controls and regulates the microfluidics, an umbilical cable comprising three pneumatic tubes and an electrical cable, a docking station and drivers. The PhysioMimix Controller can operate up to six Multi-chip plates in parallel using two Docking Stations. Each Multi-chip plate is inserted into a bespoke MPS Driver. Up to three MPS Drivers can be connected to each Docking station.
How easy is it to set up PhysioMimix OOC Systems?
System set-up is very straightforward! It should take you less than one hour. Instructions are provided within the instruction manual, or in video format to guide you through the process. No specialist engineers are required. First, install the controller on a bench or sturdy table by the incubator; then connect the cables following the colors and instructions in the instruction manual. Pass the umbilical cable into the rear (or side) port of the incubator, install the docking station into the middle shelf of the incubator and connect the cables following the same color patterns. Power on the controller. You are ready to use the system. Experimental set-up is achieved using the touchscreen on the controller unit.
Do you need a specialist incubator for PhysioMimix OOC Systems?
No, our systems are compatible with any standard laboratory incubators. One docking station fits onto the shelf of a standard incubator which makes it easy to implement in your lab. The one requirement is having a side or rear access port to connect the controller and docking station via the umbilical cable.
How do you clean and decontaminate PhysioMimix OOC Systems?
Our PhysioMimix OOC Systems have been designed with minimal maintenance requirements in mind. Each well/chip, within our consumable plates contains integrated fluid handling pumping to mimic blood flow. This all-in-one solution reduces the number of laborious and time-consuming tasks, such as the installation and maintenance of tubing, and the requirement for expensive durable pumps and flow regulators. Additionally, the design minimizes the number of routine sterilization tasks since the cells and their media remain contained within a pre-sterilized single-use consumable which should be disposed of (following Health and Safety protocol in place) at the end of an experiment.
For systems that have not been exposed to pathogens or transfected viruses, a simple wipe down with 70% ethanol will suffice. Otherwise, when the system has cultured unscreened cells or cells transfected with a virus, the system should be decontaminated with detergent/bleach and then wiped down with 70% ethanol.
How easy is it to transition from 2D culture into PhysioMimix Organ-on-a-chip assays?
It is straightforward to transition from 2D cell culture into 3D culture using our PhysioMimix OOC systems.
Firstly, the PhysioMimix system is very easy to set up and user-friendly. The hardware takes less than an hour to install and does not require an engineer to be on site. Our PhysioMimix Multi-chip consumable plates feature an open-well design that will feel familiar to anyone who is used to working with standard cell culture plates. They are ready to use as supplied i.e., Liver plates are pre-coated with extracellular matrix to promote cell attachment. This approach lowers the adoption curve versus smaller futuristic-style chips. There are some elements of assay set-up that will be new to users, such as priming the plates’ microfluidic pumps prior to cell seeding but none of these are complex procedures.
Users who are concerned about the transition to 3D when acquiring a PhysioMimix system, should be reassured that we offer online and onsite training plus a range of SOPs and “how-to” videos. To learn more about our Training and support programs, visit our support page or contact us.
Critical to the success of OOC assays is cell quality. Not all primary cells form good 3D microtissues, so pre-validating cells is hugely important. However, rather than spending your valuable time, resources, and budget quality controlling cell lots to find those that are OOC compatible we offer a catalog of 3D validated cells. Our primary cell lots have been thoroughly tested to ensure that they thrive in 3D, when cultured using our PhysioMimix systems, maintaining their function and phenotype for up to 4 weeks under perfusion. This means that you can focus on making new discoveries! This blog provides a useful overview of some of the considerations to keep in mind when validating cells for use in OOC assays.
We are also continuing to develop our range of “in-a-box” reagent kits. The first in our range is the NASH-in-a-box kit which contains everything required to recreate our in vitro NASH model in your own laboratory, including step-by step guided software protocols.
At CN Bio, the success of our users is critical to our own success. Therefore, we ensure that customers can successfully generate 3D in vitro microtissues right from the start of their journey with us. Despite the complexity of the biology that PhysioMimix OOC systems create, our new users always find the process surprisingly simple! In this blog, we explore and address some of the myths associated with OOC.
What cell types are compatible with PhysioMimix OOC Systems?
Our PhysioMimix OOC systems and Multi-chip plates have been designed to be highly adaptable to a wide range of cell types including primary cells, cell lines, induced-pluripotent stem cells (iPSC) and tissue biopsies.
Can you use animal cells in PhysioMimix OOC Systems?
Our work at CN Bio has mainly focused on combining physiologically relevant ratios of primary human cells to recreate human microtissues for drug discovery. However, the PhysioMimix OOC is an open and versatile system that can be used to develop non-human species models. When we first developed our prototype technology (the Legacy© system), with Professor Linda Griffith’s team at MIT, we used primary rat and human hepatocytes.
For some applications, such as developmental and reproductive toxicity testing, it would make sense to use animal cells since animal models are the gold standard for decision making. We are currently qualifying the use of non-human cell types, notably for Safety assessment, to show the translatability of data generated by PhysioMimix OOC to in vivo animal models.
In addition, collaborators are working with alternative species, such as the Pirbright Institute, who currently use the PhysioMimix OOC to develop avian models to study avian flu and other viruses.
The key to success is selecting the right extracellular matrix (ECM) to support the tissue/ cells of interest as well as cell culture condition optimization.
How does the PhysioMimix Multi-organ System model organ-organ interaction?
Our PhysioMimix Multi-organ System can be used for single- and or multi-organ studies. Multi-organ studies are predominantly performed using Multi-chip Dual-organ plates that contain multiple fluidically linked chambers. Cell culture medium in the well is pumped between these chambers in a manner that mimics blood flow in the body. Using this approach, we can model organ-organ interaction to discover how secreted markers from one organ will affect a second organ, multi-organ toxicity, immune-mediated toxicity or drug bioavailability.
Can you explain how Organ-on-a-chip Systems mimic tissue microenvironments?
Considerable advances have been made to culture cells to form human-like tissues at a microscale. The properties of cellular microenvironments in vivo vary from tissue to tissue and therefore different approaches are used for recreating the microenvironment of different tissue types in vitro. Depending on tissue type, the aim is to:
- enable 3D culture or co-culture of different cell types,
- expose cells to microfluidics and electromechanical cues,
- culture cells with polarity as tight barrier-like monolayers,
- deliver chemical signals,
- use engineered and functionalized extracellular matrices, among other techniques in the field of bio-microfabrication.
How does PhysioMimix OOC recreate the bloodstream via flow perfusion?
The user selects or adjusts inter- and intra-organ flow rates over extended culture periods via the touchscreen interface of the PhysioMimix OOC Controller units. If you are unsure which flow rate suits your organ model best, we can provide recommended flow rates for the organ models we have developed and validated.
The Controller unit provides precise and controllable flow via a pneumatic connection to each well/chip within our consumable plates. Each well/chip contains integrated fluid handling pumping to mimic the bloodstream. A docked plate aligns with the pneumatic lines in the system and connects with the PhysioMimix Controller. The on-plate pumps and valves are then actuated by under-plate pneumatics to create flow, as demonstrated in the diagrams below.
This approach means that PhysioMimix OOC Systems contain fewer moving parts for system reliability. Docking/undocking Multi-chip plates is a single, smooth action.
For a detailed explanation, please watch the on-demand webinar: The Rhythm of Life.
What are the benefits of perfused 3D cell culture versus static?
This topic is covered in detail within this blog and webinar. In short, recirculating fluidic flow mimics the bloodstream delivering biomechanical stimulus, oxygen, nutrients, and removing waste from 3D cell cultures. Flow perfusion enhances microtissue viability, function and phenotype and maintains cultures for up to one month.
An extensive amount of research effort has been invested into ensuring that the organ-specific flow rates delivered by our PhysioMimix Systems physiologically match that of their in vivo counterpart. Here is an example of how we calculated the flow rate for the liver: Domansky et al, 2010 during the development of the PhysioMimix in collaboration with MIT. Note that fluidic flow rates were calculated for single (Gut and Liver) organ models. When considering multi-organ models, the calculations to determine fluidic flow rate and cardiac output for each organ and between organs can be really challenging. The more organs, the more parameters must be considered. A good example of this can be found in our four-, seven- and 10-organ model publication: Edington et al, 2018).
Are the rate and direction of the flow in PhysioMimix OOC tunable?
When recreating models developed by our scientists, we recommend using the flow rates provided as these have been fine tuned to accurately recapitulate tissue exposures within the human body. However, using the Controller, you can change both the flow rate and the direction of the flow as needed to fit your experimental design.
Our pumps are optimized to work in the low µL.s-1 range as this net flow rate sits well within the requirements of our overall system design: total volumes, re-oxygenation rates and mass of tissue being perfused. We could go to significantly lower or higher net flow rates with our design, but there currently is no biological need for this.
Our PhysioMimix OOC Systems provides you with complete control at each step of your experiment, such as cell seeding, microtissue formation or dosing. This is particularly enabling should you wish to develop a new model using our Single-, or Multi-organ Systems. When developing multi-organ models using our Dual-organ plate, users can optimize both the inter-organ flow rate, for accurate on-platform pharmacokinetics, and intra-organ flow rate is adjustable to optimize oxygen and nutrients intake as well as mechanical forces.
Apart from flow, which other features do you consider as critical/ key to successfully developing organ-on-chip technologies?
Other areas to focus on include the physical and chemical properties of the tissue scaffold (biocompatibility, geometry, surface hardness, wettability) and the chemical/biochemical makeup of the media. These are the things each tissue experiences in vivo and are therefore important for the experiment. However, to develop a commercially accessible a product, there are many further aspects to consider, some of the most important include usability (easy/fun to use, access to the tissue/media for sampling, preventing infection/contamination), throughput (how many replicates can be simultaneously run), manufacturability (reproducibility, quality control, sterilization methods, packaging, shelf-life) and there are regulatory aspects too as any product needs to be proven safe and effective. So overall, it is a very multidisciplinary task.
Can PhysioMimix OOC systems be used to screen new drug modalities?
Our PhysioMimix systems are generally agnostic to drug type, providing compatibility with small, or large molecules and newer drug modalities. In a recent Application Note, we assessed the drug-liver injury (DILI) potential of two antisense oligonucleotides (ASOs) using our liver-on-a-chip model. Collaborators have also used our liver model to investigate the uptake and distribution of ASOs within liver tissue, click here to read more.
The advantage of OOC technology in this area is that they provide a humanized test system that can be used to test drugs with human-specific modes of action. There is much interest in OOC’s potential in this area due to the spiraling costs, and accessibility limitations and ethical concerns surrounding the use of non-human primates. A more comprehensive overview of this topic is provided in this recent article written for Drug Discovery World.
Is it possible to use PhysioMimix OOC Systems for therapeutic gene delivery?
OOC/MPS can be used for testing genetic engineering therapeutic agents, including adenovirus-associated viruses (AAV) and adenovirus, ASOs, small interfering RNAs (siRNAs) and CRISPR agents. The longevity of MPS cultures makes them particularly well suited to studying these agents which need some time to establish in cellular systems before having an efficacy or toxicological effect. Additionally, as most of these agents are human cell-specific, they can be tested on primary human cells cultured in MPS/OOC.
How many organs can you culture using the PhysioMimix Multi-organ System?
The DARPA body-on-a-chip program that we completed in collaboration with The Massachusetts Institute of Technology (MIT), set the ball in motion towards the development of complex and interconnected systems using four-, seven- and 10-interconnected MPS (four, seven and 10 organs. Edington et al, 2018). It was a very exciting endeavor and an enormous undertaking which uncovered many challenges in terms of scaling and interconnection that are still being worked through and optimized for commercial settings. For example, discovering a common medium for all interconnected organs is a necessary step, but developing a universal solution that maintains the health, phenotype, and function of so many organs is far from a simple task in practice.
Additionally, the more organs that are interconnected, the more complex the system microfluidics becomes. Ideally, system microfluidics should recreate a physiologically relevant flow rate and cardiac output within each organ as well as between each organ in the system. On top of that, the solution needs to be easy to operate with the ability to plug and play different organ combinations, a prerequisite for an easy integration into laboratory workflows. To provide user-friendly workable solutions for use in commercial settings, we have stepped back from the full body-on-a-chip of DARPA days and have taken our time to develop gold-standard single-organ and dual-organ models to form less extensive but more usable multi-organ systems that provide the next dimension of safety and drug bioavailability in vitro data.
By connecting liver tissue with another “route of entry” organ (gut/liver or lung/liver models), PhysioMimix users can now study reactive metabolite-driven toxicity whilst simultaneously evaluating drug absorption, metabolism, and bioavailability.
The more we develop and understand complex models, the easier it will be to develop body-on-a-chip models for applications such as personalized medicine, but this is not something we see happening in the near future. Although CN Bio’s vision is to become the first commercialized body-on-a-chip provider, we currently see a greater value in developing a broader portfolio of applications for single- and dual-organ models which will enable end-users, particularly in the pharmaceutical industry but also wider industries such as cosmetic or food, to benefit from improved data translation over standard techniques.
How complex is it to generate a common media that is beneficial for two inter-connected organs, or tissues?
The complexity greatly depends on the cell types used, the two-organ model set-up and the application. For a barrier-liver model set-up, such as our Gut/Liver model for ADME assays, a slightly optimized version of the media can be used. Initially, each organ is cultured individually until both tissues are fully formed. Gut cells are seeded and grown on the apical side of a Transwell® insert and liver cells are seeded directly into the liver chamber of the Dual-organ plate. Once the organs are cultured, flow between the compartments is activated and drugs are added, however, the gut’s media, remains separated by the Transwell insert, its membrane and the physical barrier of the gut microtissue. Only drugs that are absorbed by the gut transfer to the basolateral side of the chamber and into the media that supports liver function. However, if two organs are both initially seeded into the Dual-organ plate, one common media would be required to maintain the health, phenotype, and function of both organs which would require some optimization. When optimizing media, the end application must be considered. For example, serum is usually used to culture Caco-2/goblet cells, however, for PBPK studies we use a serum-free gut media to reduce the risk of the drug binding to serum proteins. Gut cells tolerate this remarkably well with no drop in epithelial barrier integrity (TEER).
What endpoint markers can you measure from PhysioMimix Organ-on-a-chip assays?
Thanks to the large sampling volumes of the system (up to 1 mL of media and relatively large 3D tissues grown on scaffolds, or Transwell® inserts), a wide and diverse set of end-point parameters can be measured from each sample – either by repeat sampling over time and/or at the termination point of the experiment. These include microscopy, histology, biomarker expression, metabolite profiling, toxicity profiling and -omics analysis.
Please visit individual application pages for a list of commonly derived endpoint measurements.
Can you combine pharmacology and toxicology studies at the same time with PhysioMimix Multi-organ System?
Absolutely yes! The PhysioMimix Multi-organ System’s Dual-organ plate provides a large volume of media (up to 1 mL) and tissue from which to sample, enabling pharmacological and toxicological studies to be combined. For example, using the Gut/Liver model to mimic in vivo drug transit conditions, on-board pharmacokinetic profiles for the drug can be generated. The exposure of the target tissue to a dynamic concentration of the drug’s metabolites can then be assessed, from which any toxic effects can also be determined.
How comparable are the results from microphysiological systems versus outcomes in clinical trials?
Organ-on-a-chip (OOC), or Microphysiological systems (MPS), are fast developing technologies that can be used for various applications within the drug discovery and development process. The evaluation of these technologies versus specific contexts of use is ongoing and involves comparison to preclinical and clinical data sets to understand translation between these complex in vitro models and humans. Overall, a cellular microenvironment with physiological properties can prolong, enhance, enable, or stabilize the function of tissue-specific cells relative to traditional culture systems. The next step is evaluating how well OOC/MPS models predict clinical results. For this purpose, OOC systems should be tested for specific contexts of use (COU) using assays and compounds (with controls) that are related to each context of use.
In benchmarking studies, we compare our organ-on-a-chip data against clinical data for reference compounds. Tsamandouras et al, 2017 is a great example. In this collaborative study with Prof. Linda Griffith’s lab at Massachusetts Institute of Technology (M.I.T.), various well-known drugs (e.g.: lidocaine, ibuprofen), were investigated to establish translatability between the OOC model and human hepatic drug metabolism This study also successfully predicted the associated in vivo population variability by exploring donor-donor variability in in vitro OOC models too. A second example is Sarkar et al 2017, who published their integrated assessment of Diclofenac biotransformation, pharmacokinetics, and omics-Based toxicity in a three-dimensional human liver-immunocompetent co-culture system. These and other publications are cited in this article written for Drug Discovery World.
This area is also being explored in more detail by our collaborators at the US FDA too. Collaborations with the FDA began in 2017, to evaluate our PhysioMimix OOC system for investigating critical drug parameters including metabolism, toxicity and drug-drug interactions. Concluding in 2021, the FDA published a paper outlining the accuracy and reproducibility of the liver-on-a-chip model, alongside its enhanced performance over conventional techniques.
Building on success, our partnership has since expanded twice– in May 2021 and January 2023, further demonstrating the value of human predictive single-and multi-organ models within the drug discovery and development space.
In 2021, the expansion sought to further investigations into CN Bio’s liver-on-a-chip, as well as opening a new project to assess our lung-on-a-chip model for studies involving inhaled drug products.
Most recently in 2023, research turned to CN Bio’s multi-organ MPS, aiming to investigate the accuracy of the PhysioMimix Multi-organ System for human drug bioavailability studies, with a goal to better inform dosing regimens to improve efficacy and limit side effects.
Please also read our Application Notes for more examples of benchmarking studies versus specific contexts of use.
How do you account for scaling of organs, in microphysiological systems?
We use a variety of scaling methodologies and do not have a one-size fits all approach. Most commonly, we focus on a functional methodology to scale microtissues to ensure crosstalk between organs is physiologically relevant, but other methods are available. For example, to scale and extrapolate in vitro data generated from our PhysioMimix Multi-organ System into an in vivo prediction, we use physiologically-based pharmacokinetics (PBPK) modeling. By comparing the behavior and toxicity of a compound in our Gut/Liver MPS to PBPK, we provide users with a physiologically relevant picture of a drug’s behavior and the crosstalk between gut and liver tissues. To know more, read our multi-organ application note: Drug metabolism in a gut-liver microphysiological system.
Collaborators at Roche have also used this methodology in their recent publications (Milani et al 2022 and Docci et al., 2022).
Have you quantified cost and time savings in drug development using PhysioMimix OOC?
This has not been fully quantified by CN Bio, but has been explored by Franzen et al., in 2019.Their independent research estimates fully integrating OOC into pharma workflows could save up to 10- 26% of all R&D costs: $49bn a year, driven by changes in direct costs, success rates and the length of the R&D process. They predict that the most impacted phases are the lead optimization and preclinical phases of R&D.
At CN Bio, we are working on our own package of information to highlight the potential economic benefits of OOC but first we have an ongoing temperature checked the market to make sure that we are aligned with customer needs and what we can do to ease their path to adoption.
The first output from this project is a 2-page document, which summarizes the highlights of a KOL panel discussion and market research survey results (125 respondents completed in early 2023 by the Pharma Intelligence group, Citeline), which can be downloaded here. Also, you can access the full survey results here.
Can Organ-on-a-chip potentially replace, or reduce animal use?
Before answering, it is important to highlight that OOC/MPS models were not originally designed to replace animal models but to provide researchers with an investigational tool to use alongside current in silico, in vitro and in vivo models to address the translatability limitations of preclinical assays, including animals. Our aim for OOC is to reduce the number of test compounds that require in vivo animal studies by deriving better insights ahead of time, such that only the very best drugs make it through and their ADME/Tox profiles are well defined. As a result, the number of animals required to test them has lessened. Should there be a time when superior-performance is proven, we hope that OOC models will eventually replace animal use This point is explored in more detail in this Medicine marker article.
Also, as new human-specific drug modalities continue to emerge, the need for more predictive preclinical models is more important than ever. Coupled with increasing awareness around ethical considerations and the spiraling cost of animal experimentation, there is growing interest in the development of New Approach Methodologies (NAMs), such as OOC, that enable researchers to design more predictive and cost-efficient in vivo studies that reduce the number of animals required. Recent ground-breaking legislature, including the US Government’s FDA Modernization Act has opened the door to more widespread NAM adoption.
The U.S Food and Drug Administration (FDA) has also recognized the potential for MPS to improve the predictability of drug discovery. Through collaboration, the FDA is evaluating the performance of PhysioMimix OOC models versus gold standard techniques. In a recent publication (Rubiano et al., 2021), the superior performance of the PhysioMimix’s Liver-on-a-Chip model for applications, that include drug metabolism, safety, and accumulation over traditional approaches. The data within this study provides decision-makers with the collateral to justify adoption of MPS into their workflows and by adopting these powerful systems, we offer an option to help, reduce and refine the number of animal tests with a view to their future replacement. The FDA has since expanded their collaboration with us (in 2021 and, 2023), to explore additional single-and multi-organ models and their specific contexts of use, including our lung-on-a-chip model for studies involving inhaled drug products and multi-organ models for human drug bioavailability studies.
The study of fatty liver disease represents another area where improved models are desperately needed as animal models do not adequately represent the pathophysiology of complex human metabolic diseases such as Non-alcoholic fatty liver disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH). This topic is explored in detail in this blog and article. By comparison, our PhysioMimix NASH model demonstrates an improved alignment of transcriptomic profiles compared to human data and more closely replicates changes found in NASH patients than the murine WD model (Vacca et al., 2020), improved pathophysiology that more accurately recapitulates long-term fibrotic and inflammatory NASH phenotypes (Kostrzewski et al, 2019) versus traditional approaches and delivers more clinically relevant responses in response to therapeutic challenge. If you’d like to know more about this model please visit our application page, watch our on-demand webinar in collaboration with AstraZeneca.
Our collaborators at Charles River Laboratories have developed a cost-effective Liver MPS model using the PhysioMimix that provides a reliable assessment of a compound’s genotoxicity in the human liver compared to gold standard animal models. If you’d like to know more about their work, you can watch their on-demand presentation from SOT 2023.
Additionally, for safety assessment applications, it would make sense to recreate species specific rat, dog, and non-human primate OOC models, since animal models are the gold standard for decision making. We are currently qualifying the use of non-human cell types to show the translatability of data generated by PhysioMimix OOC to in vivo animal models.
We also work collaboratively with organizations’ such as The North American 3Rs Collaborative(NA3RsC) and Animal Free Research UK (AFRUK) to drive awareness for OOC/MPS technology as an animal alternative.
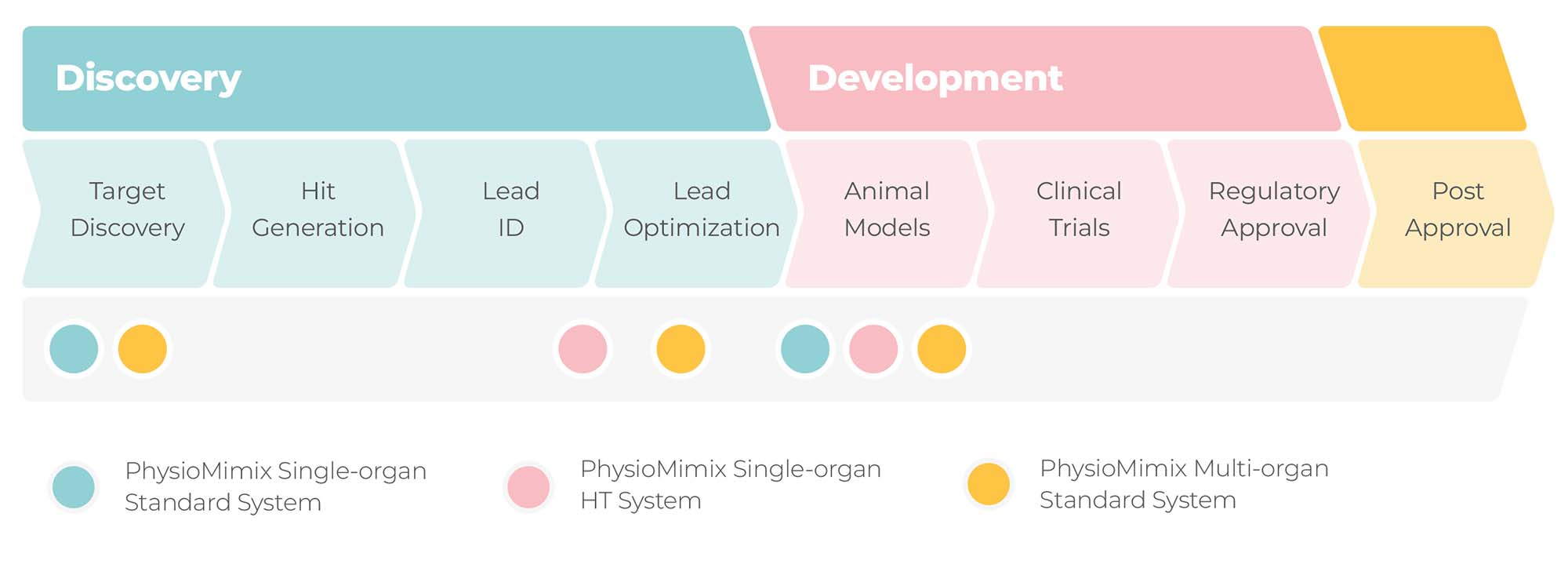
For what purposes and at which stages of drug discovery and development can Organ-on-a-chip be used?
The topic of where and for what purposes Organ-on-a-chip (OOC),otherwise known as Microphysiological Systems (MPS) can be used is explored in detail in these articles written for International Biopharmaceutical Industry and Drug Discovery World. In summary,
- Organ-on-a-chip (OOC), otherwise known as Microphysiological Systems (MPS), can be used right at the beginning of drug discovery to identify and validate targets in models that more accurately represent human disease.
- Through the generation of human translatable safety, efficacy and ADME data, OOC present an opportunity at the lead optimization stage to re-engineer potentially flawed drugs early to maximize their chance of success once they hit the clinic.
- In the drug development phases, OOC can be used ahead of animal studies to test larger numbers of conditions/refine conditions/minimize the number of animals required for safety, efficacy and ADME studies.
- OOC can be used alongside animals to confirm or query results, reducing the risk of unforeseen issues due to interspecies differences.
- In certain scenarios, OOC provides an animal alternative, minimizing the unnecessary use of animals where translatability to humans is poor, i.e., where preclinical animal models do not exist (e.g., Hepatitis B virus (HBV) infection), or for the testing of drugs with human specific modalities which require testing using non-human primates, the latter of which are extremely costly, ethically undesirable and challenging to source.
The illustration below shows the drug discovery and development stages that our PhysioMimix OOC range of microphysiological systems are most suited.

Additionally, recent market-led information regarding this topic can be found in this document summarizing the highlights of a KOL panel discussion and market research survey results (125 respondents completed in early 2023). You can access the full survey results here.
Can Organ-on-a-chip data be submitted to regulatory authorities for IND filing, or other global regulator equivalents?
Yes, recent ground-breaking legislature, including the US Government’s FDA Modernization Act has opened the door to more widespread New Approach Methodologies, also known as New Alternative Methods, or NAMs, which include Organ-on-a-chip.
The impact of the FDA Modernization Act 2.0 is explored in detail in this blog, however the key take home messages are that the federal mandate stipulating that drug testing in animals MUST be performed, has now been eliminated. The use of NAMs to establish drug safety and effectiveness may be adopted in place of animal testing where appropriate.
In Section 3209 of the FDA Modernization Act 2.0 entitled “Animal Testing Alternatives,” the bill amends the regulatory guidance at the FDA that requires animal testing for drugs and biosimilars. The bill amends the Federal Food, Drug, and Cosmetics Act (FFDCA) to:
- Substitute the term “nonclinical tests” for the current “preclinical tests” (including tests on animals),
- Substitute the term “animal” for “nonclinical tests” and,
- Add a new section defining “nonclinical tests” to include human-relevant testing methods such as cell-based assays, microphysiological systems (such as Organ-Chips), or bioprinted or computer models.
It’s important to note here that, although the U.S. has been the first regulator to take the leap, and North America in general are leading the way, important work is going on in other regions to drive change too as regulators acknowledge that animal testing for the sake of animal testing is not the way forward. Back in 2014, here in the U.K., plans were announced to reduce the use of animal tests in scientific research, aiming to replace those tests with ‘scientifically valid alternatives’ where possible. More recently in 2021, the European Parliament voted in favor of plans to phase out animal testing in research.
In 2022, CEN, the European Committee for Standardization who are focused on helping to drive the adoption of new technologies set up a focus group on OOC and not-for-profit groups such as The European Organ-on-a-Chip Society (EUROoCS) have been growing their network since 2018 in a bid to share and advance knowledge in the field towards better health for all.
However, OOC is still a developing field and there are some challenges that need to be addressed before its data is widely accepted by regulatory agencies. These include demonstrating the reliability and predictive power of OOC versus specific contexts of use, standardization regarding how OOC models are developed and validated and guidance regarding how to use OOC data in regulatory contexts.
Behind the scenes, companies like ours have been developing technology, the advanced single- and multi-organ models and their applicability (within the disease modeling, toxicity testing and ADME space) for over a decade. Through collaboration with academia, drug discovery companies, consortia (such as the IQ Consortium) and various regulators (including the FDA) we have collectively been growing a body of evidence to demonstrate the ability of these human-relevant models to deliver data that better predict human outcomes, and to overcome the aforementioned challenges.
If you do not find the answer to your question listed, please contact us