How to more accurately estimate human drug bioavailability?
Estimating the bioavailability of drugs in humans during preclinical development is necessary to set safe and effective doses in the clinic. Submitting bioavailability data is also a regulatory requirement.
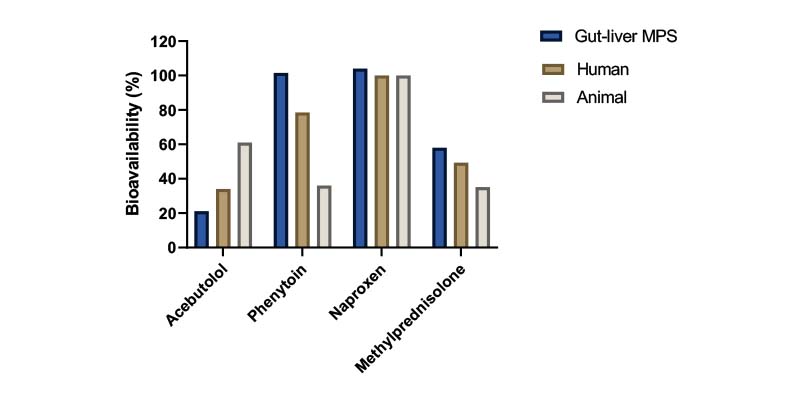
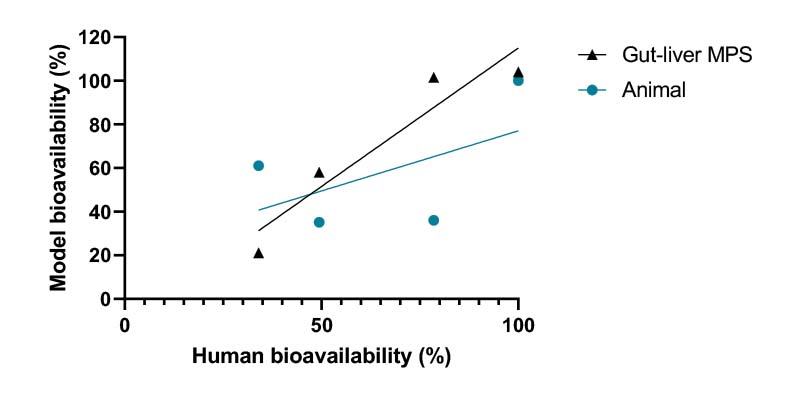
Extrapolating PK parameters from animals to humans during drug development is challenging. The overall correlation between animal and human bioavailability is poor mainly due to differences in physiology and metabolic capacity.
Achieving high oral bioavailability is advantageous to reduce safety issues. Insufficient bioavailability in humans requires an increase in drug dose to be effective, however, this increases the risk of toxicity and side effects.
Our solution
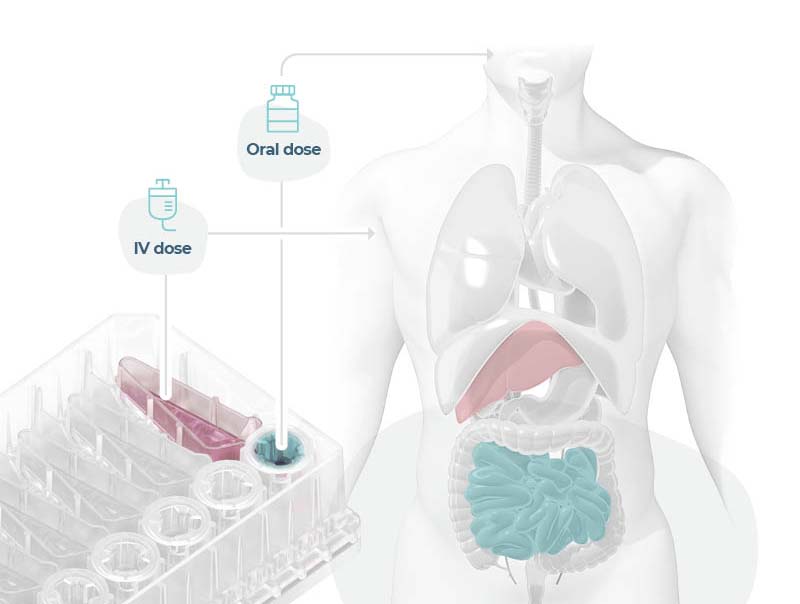
We have developed a PhysioMimix® Bioavailability Assay that uniquely recreates the combined effect of intestinal permeability and first-pass metabolism using PhysioMimix Gut/Liver-on-a-chip models. Our advanced in vitro human models recreate the function and fluidic interaction of the human gut and liver to emulate the dynamics of drug absorption through the intestinal barrier followed by their subsequent metabolism by the liver. The approach enables you to compare intravenous and oral compound dosing for the prediction of human oral bioavailability.
The assay delivers robust and reliable data to better inform the design of in vivo animal studies and validate in silico PBPK modeling. Its insights facilitate the improved estimation of preclinical bioavailability for more informed decision-making ahead of clinical trials.
Take a tour with our bioavailability animation
Studying drug bioavailability
Limitations with current techniques
- Animal models fail to accurately predict human bioavailability
- Animal models have varying expression levels for enzymes that drive drug metabolism in humans
- Simple in vitro models cannot model the systemic effects of the drug
- In silico models are dependent on quality input parameters that rely heavily on early in vitro and animal studies
Advancements with PhysioMimix OOC
- Enables the direct, accurate measurement of bioavailability using a human-relevant system
- Perfusion of microtissues promotes the metabolic capacity of cells
- Allows the combined effects of intestinal permeability and liver metabolism to be explored
- Data derived using a human-relevant approach better informs in-silico models
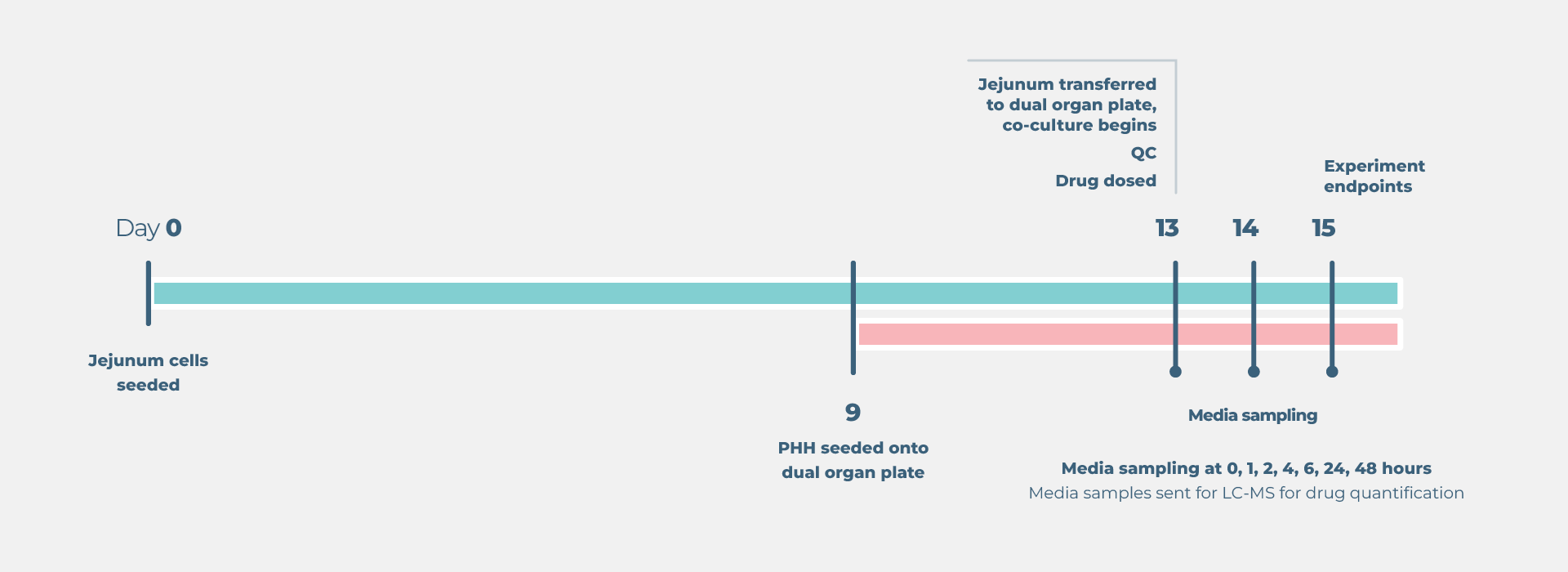
End point measurements
Longitudinal and endpoint measurements include (but not limited to):
Liver: Functionality biomarkers
- Cytochrome P450 enzyme activity
- Lactose dehydrogenase (LDH) release
Gut: Functionality biomarkers
- Trans epithelial electrical resistance (TEER)
- Lucifer yellow or dextran permeability assay
- Lactose dehydrogenase (LDH) release
Profiling analysis
- Media samples send to LC/MS for bioanalysis
- Parent drug concentration over time
- Profiling bioavailability
- Metabolite concentration over time
- Area under the curve estimation
Optional profiling analysis
- Microscopy biomarker analysis
- Transcriptomics
Modeling an orally administered drug’s route of entry
Uniquely recreate the combined effect of intestinal permeability and first-pass metabolism in vitro using human Gut/Liver-on-a-chip models to more accurately estimate oral bioavailability.
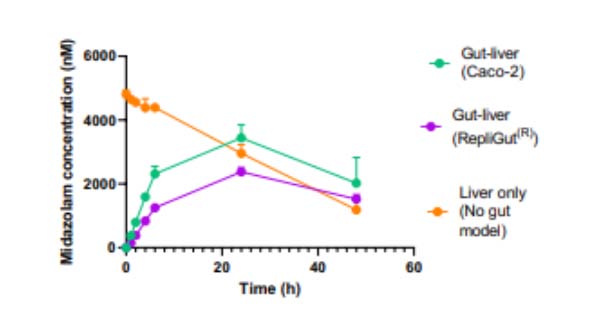
In this example, we demonstrate Midazolam metabolism by the liver in isolation versus the combined contribution of both organs using two Gut/Liver-on-a-chip models (Caco-2, or primary human RepliGut®).
Midazolam concentration in the dual-organ model was determined over time following either apical dosing of gut tissue (oral) or in liver only wells (IV) and sampling media from the liver compartment. Improved estimations were observed using the primary RepliGut/Liver model.
Improve bioavailability estimations with a complementary in vitro approach
The PhysioMimix Bioavailability assay simulates oral and IV dosing regimens to improve the accuracy of human bioavailability estimations compared to animal studies. Data derived from the assay can be used to better inform in vivo study design.
Access our ADME Service
Get instant access to PhysioMimix Bioavailability Assay via our CRO Service. Through a collaborative approach, our experts work with you to plan and execute your study.
Standard and bespoke projects are carried out by our dedicated team of scientists in our CRO facility providing you with actionable data within weeks.
Add PhysioMimix OOC into your lab
Harness the power of PhysioMimix OOC in your own lab with the purchase of a Multi-organ microphysiological system.
With a growing community of users and support from our experts, there has never been a better time to transition into 3D cell culture.