A unique opportunity to measure drug ADME in vitro without using animals
Our in vitro assays are highly predictive of clinical results. Studies investigating drug metabolism, metabolite identification, permeability, and bioavailability are conducted using human highly functional, metabolically competent single- and multi-organ models.
Service overview:
We offer a range of single- and multi-organ models to derive human-relevant data to better inform the selection of lead candidates with desirable ADME properties.
Through this service, you can utilize a combination of our expertise and highly metabolically active, long-term cultures to derive unique in vitro data.
- Hepatic clearance including low clearance compounds and metabolite identification, including Phase I and II metabolites
- Gut metabolism and permeability
- Lung permeability in upper and lower airway models
- Bioavailability estimation using multi-organ models to simulate route of administration
- Method developed with protein-free cell culture medium in conjunction with low non-specific binding multi-chip assay plates
Customer feedback
Working with CN Bio has been a pleasure, the team listened to our non-trivial requirements and made useful suggestions to help meet objectives. During the course of the project, the team were responsive and helpful. The results were delivered as agreed and helped us to move towards our goals.
Dr Giuseppe Ferrandino
Senior Translational Scientist at Owlstone Medical
View more PhysioMimix reviews on SelectScience

How the service works:
Utilizing our PhysioMimix OOC systems allows the modeling of human ADME profiles in liver, gut, lung, or multi-organ combinations.
This in vitro service generates novel insights into the human body’s effect on drugs, previously only possible using animal models.
Your dedicated contact will work collaboratively with you from the start to the end of the project.
- Design and finalize experiment plan
- Customer supplies required amount of drug(s)
- Growth and preparation of organ models prior to treatment – between four and 21 days, dependent on assay selected
- Compound dosing and sample collection up to four days depending on the model.
- One to two weeks to run endpoint assays, analyze data, and complete the report
- ~ two months to complete the study from receiving order
- Media samples sent to customer, or third party for LC-MS analysis
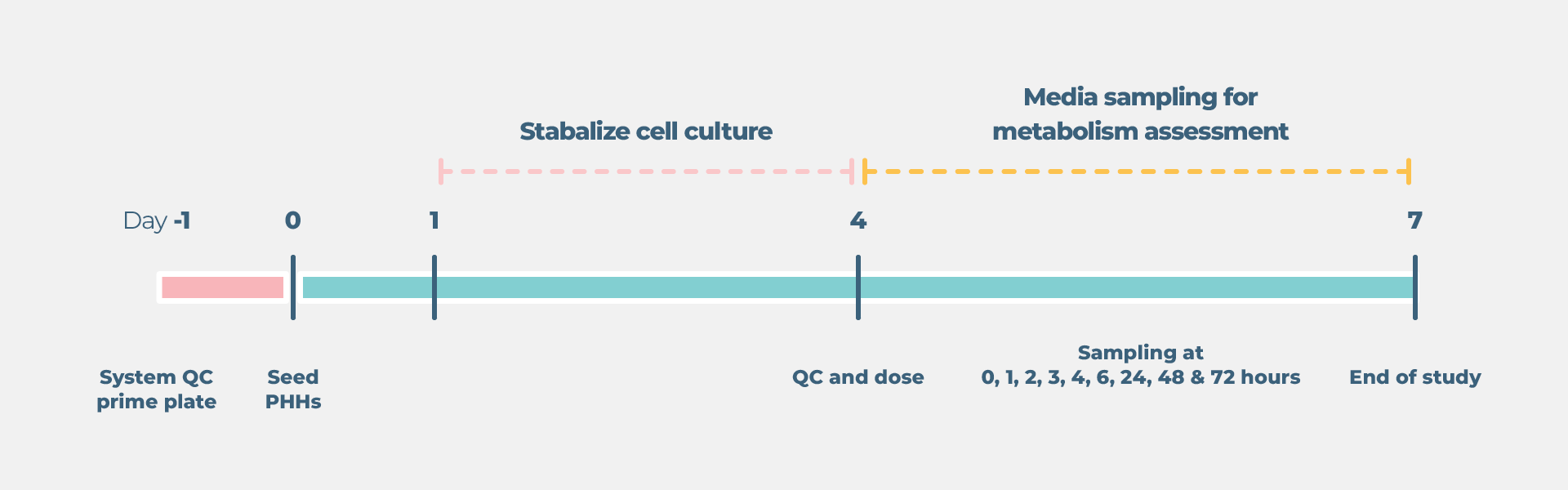
Standard liver drug metabolism cell culture timeline
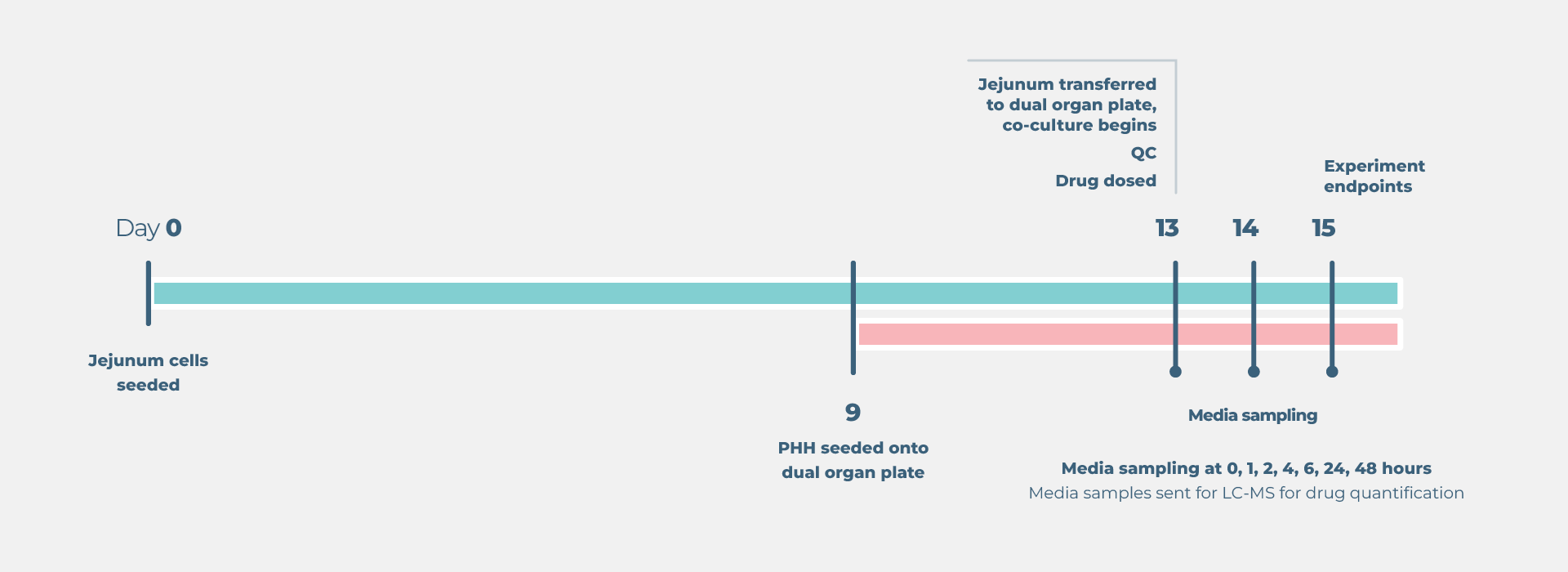
Standard gut/liver drug bioavailability cell culture timeline
Endpoint measurements
Included, but are not limited to:
Functionality biomarkers
Liver
- Cytochrome P450 enzyme activity
- Albumin production
- Urea production
- Lactose dehydrogenase (LDH) release
Gut, or Lung
- Trans epithelial electrical resistance (TEER)
- Lactose dehydrogenase (LDH) release
Profiling analysis
Liver
- Media samples ready for LC/MS analysis
- Cytochrome P450 enzyme activity
Gut, or Lung
- Media samples ready for LC/MS analysis
Related applications
Drug absorption
Our co-culture gut and lung absorption assays provide in vivo-like biological barrier properties to study compound absorption rates and more closely predict human outcomes.
Drug metabolism
Our liver, lung, and gut in vitro models can be used separately or in combination to study drug metabolism. These stable, human models accurately mimic the complexity of the physiological environment and offer a major advance for studying DMPK.
Drug bioavailability
Our multi-organ oral bioavailability assay with connected gut and liver models can provide accurate estimations of human bioavailability early in drug development, improving the chance of success in clinical trials.