How can you improve human hepatic drug metabolism predictions?
Drug metabolism is a complex process where drugs are structurally modified to form metabolites. Studying drug metabolism and pharmacokinetics (DMPK) is required to identify lead compounds with optimal PK/PD properties, optimize efficacy, and minimize safety issues.
Standard in vitro drug metabolism and pharmacokinetics studies face various challenges. Many are incompatible with new therapeutic modalities, are inaccurate predictors of human in vivo clearance rates, and fail to identify rare or human-specific metabolites.
Our solution
Individual Liver-, Lung-, and Gut-on-a-chip in vitro models can be used to study drug metabolism, metabolites, and transporter-mediated uptake and efflux.
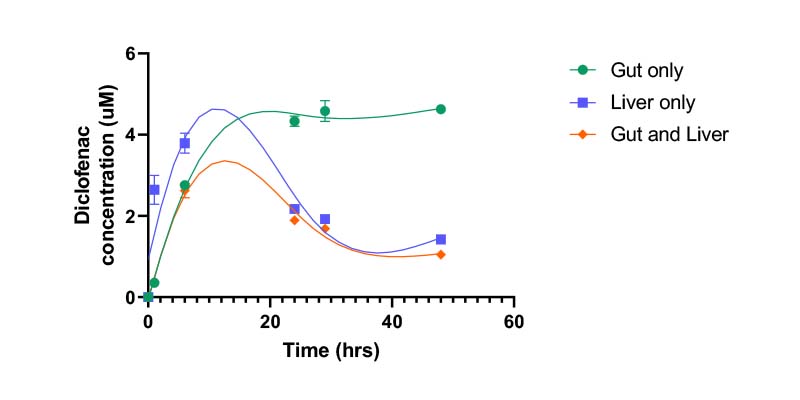
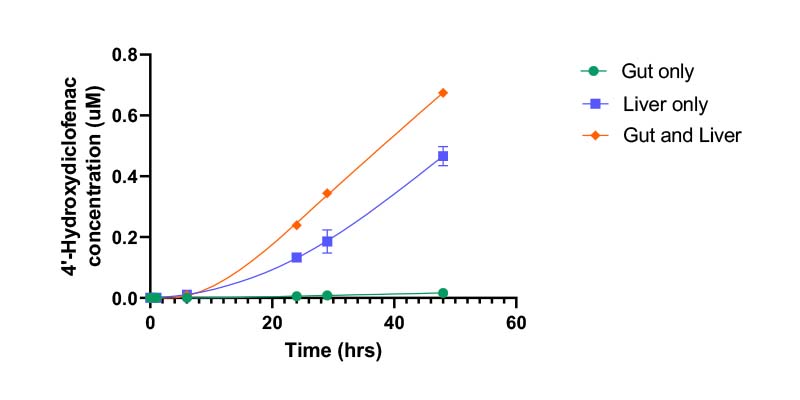
Connecting two models related to DMPK offers an improved prediction of in vivo pharmacokinetics and pharmacodynamics. For example, combining liver and gut models allows orally administered drugs to be studied in a multi-organ system. The system can reflect compound permeability through an intestinal barrier, hepatic metabolism, organ–organ interaction, and crosstalk.
PhysioMimix® Drug Metabolism Assays allow the measurement of parent and metabolite concentrations within the intracellular compartment and culture medium, to determine all standard metabolic parameters. They are particularly well suited to measuring slowly metabolized, low clearance drugs where in other systems, such as suspension hepatocytes, compound turnover is not detected.
Our organ-on-a-chip (OOC) models maintain phase I and II metabolism together with transporter function at in vivo-like levels for weeks. The ability to recirculate media around the cultures allows for the decrease in parent or build-up of metabolites to be measured and complete metabolic characterization from a single culture.
Studying drug metabolism
Limitations with current techniques
- Low clearance drugs can’t be evaluated with suspension hepatocytes
- Many systems do not show adequate phase II metabolism
- Metabolism is not measurable in a ‘single pass’ OOC and spheroids
- Some OOC technologies are unsuitable as they use Polydimethylsiloxane (PDMS) which absorbs drugs
Advancements with PhysioMimix OOC
- Prolonged metabolic competence means low clearance drugs can be evaluated
- Liver-on-a-chip provides phase I, II, and tertiary metabolism
- Our models have an optimized cell-to-medium ratio, ensuring measurable metabolism
- Our systems are made from low-binding non-silicone materials
- Liver-on-a-chip models typically contain 500K cells, providing material ‘horsepower’ for rare metabolite detection
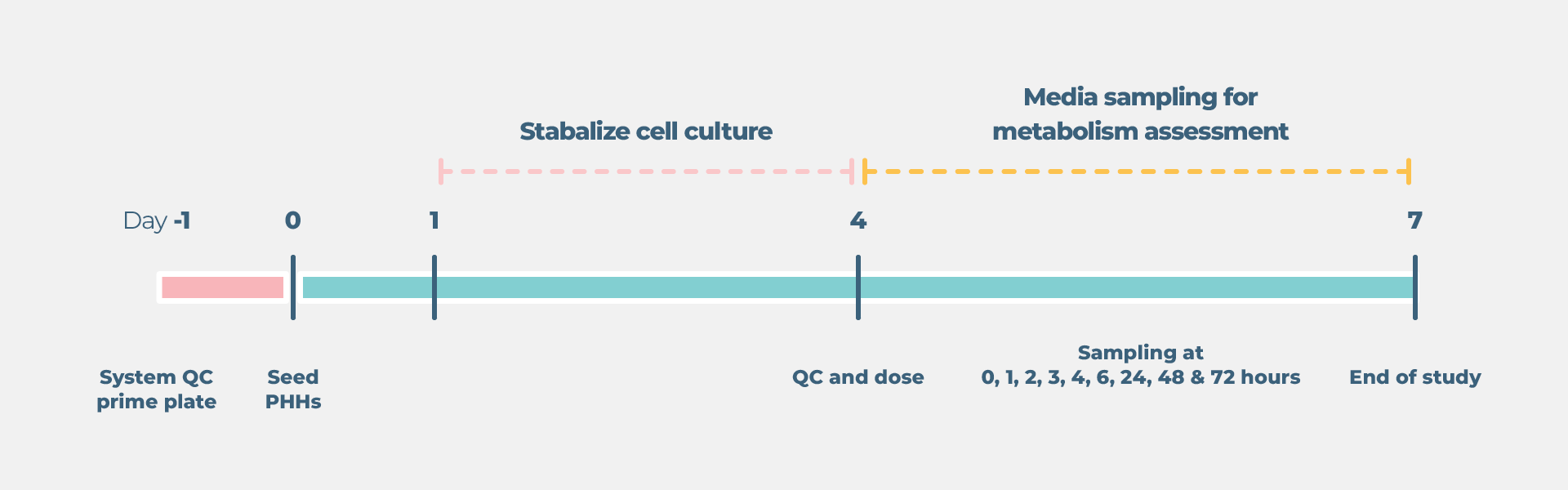
Cell culture timelines
End point measurements
Longitudinal and endpoint measurements include (but not limited to):
Functionality biomarkers
- Cytochrome P450 enzyme activity
- Albumin production
- Urea production
- Lactose dehydrogenase (LDH) release
Profiling analysis
- Metabolite production over time
- Media samples send to LC/MS for bioanalysis
- Drug concentration over time
- Parent drug clearance
- Known metabolite production
- Area under the curve estimation
Optional profiling analysis
- Quantitative PCR
- Transcriptomics
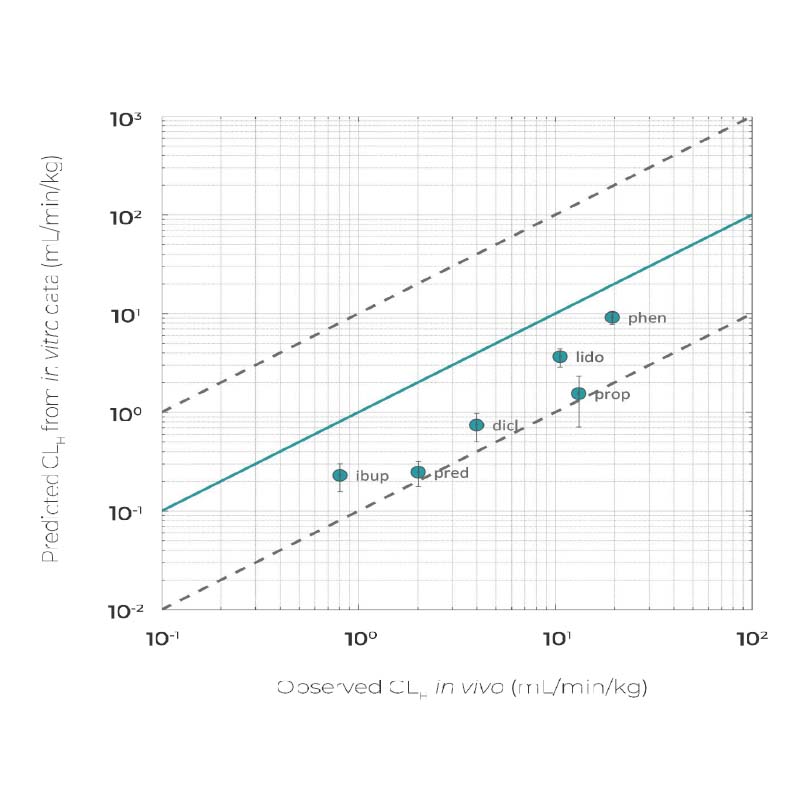
Predict slowly metabolized drug clearance rates
From slow to fast, a range of in vivo drug clearance rates can be accurately predicted using our metabolically competent, human-relevant in vitro Liver-on-a-chip.
Access our ADME Service
Get instant access to PhysioMimix Drug Metabolism Assays via our CRO Service. Through a collaborative approach, our experts work with you to plan and execute your study.
Standard and bespoke projects are carried out by our dedicated team of scientists in our CRO facility providing you with actionable data within weeks.
Add PhysioMimix OOC into your lab
Harness the power of PhysioMimix OOC in your own lab with the purchase of a single- or multi-organ microphysiological system.
With a growing community of users and support from our experts, there has never been a better time to transition into 3D cell culture.