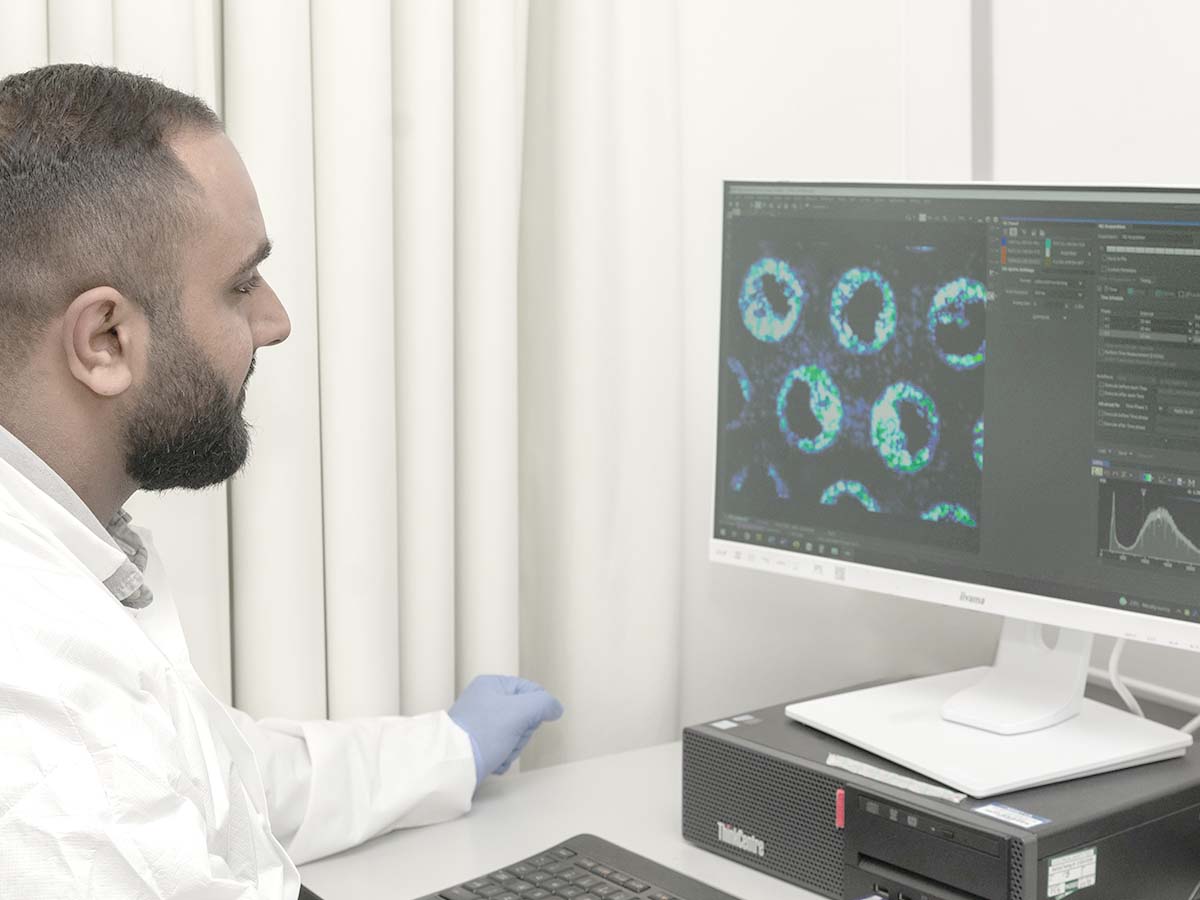
Single and multi organ-on-a-chip (OOC) models
Organ-on-a-chip models (also known as microphysiological systems) bridge the gap between traditional cell culture, animals, and the clinic by more accurately replicating human physiology in vitro to overcome the relevance limitations of current approaches.
Explore our models

Single organ-on-a-chip
Single-organ models allow detailed investigative studies of individual organ function and modeling of specific disease states to recreate intricate human physiology in the laboratory.
Liver-on-a-chip model
The liver is a key organ in the study of drug metabolism and safety toxicology. Understanding how a drug is processed by the liver, discovering adverse effects and informing dose setting, are crucial to drug development.
Rates of liver disease are also on the rise, driven by alcohol consumption, diabetes, obesity rates, and infection. Some remain unmet–or poorly met–therapeutically. More human-representative models are required to address these concerns.
Our Liver-on-a-chip is designed for the study of drug metabolism, toxicology, and liver diseases.
- Easily identify liver toxicity markers, model liver disease, and study drug metabolism
- Extended culture lifespan (> four weeks) enables the study of acute and chronic disease and drug dosing
- Replicates liver disease with a high degree of accuracy providing a suitable system for studying signaling pathways
- Detect phase II metabolites and drug accumulation to support clinical data interpretation
- Combine hepatic and immune cell types to identify adverse drug effects across a broad range of modalities including monoclonal antibodies, oligonucleotides and traditional small molecules
Gut-on-a-chip model
Diet and lifestyle have a direct impact on gut health. The gut plays a complex role in maintaining health and performs a range of functions from transportation, absorption, and metabolism to playing a part in the immune response.
Understanding its physiology and pathology can inform drug pharmacology. Our Gut-on-a-chip is cultured from a mixture of epithelial and goblet cells to enable studies of drug absorption, barrier integrity, and inflammatory disease by modeling the physiology of the gut epithelium.
- Under perfusion, a complex 3D-like morphology forms that closely models human gut physiology
- Absorptive functions develop from increased tissue depth and greater tissue maturity
- It exhibits a biological barrier function with permeability that is aligned to that of the human gut
- The model expresses tight junctions and secretes mucus
Lung-on-a-chip model
The lung is a key barrier organ, useful as a route of administration for inhaled medication. It is also susceptible to a variety of diseases caused by environmental exposure to substances. Mimicking the physiology of the human airway enables the study of drug absorption, infection, and toxicity.
Chronic respiratory diseases are some of the most common non-communicable diseases due to the presence of noxious inhaled materials around us.
We offer two human lung models (bronchial and alveolar) that capture the tissue microarchitecture of different lung areas and display their specific functions.
- Dynamic perfusion induces shear stress and enhances microtissue formation, promoting in vivo-like functionality
- The models reflect the complexity of lung physiology allowing translational studies of pulmonary disease and toxicity
- The combination of an endothelial and epithelial layer of primary human cells mimics tissue-specific features
- An air-liquid interface facilitates the effects of air-borne agents or inhaled medications to be studied
Multi organ-on-a-chip
Multi-organ models give detailed information on organ-to-organ crosstalk, pharmacokinetics, and biodistribution. They allow drug effects on the targeted organ, as well as off-target effects on other organs, to be tested.

Gut/Liver-on-a-chip model
Investigation of the ADME properties of a drug is essential during the early stages of drug development. Current approaches inadequately predict human clearance rates, miss rare or human-specific metabolites and many are incompatible with new therapeutic modalities.
As a result, drug attrition in Phase II and III clinical trials is now mainly due to a lack of clinical efficacy rather than toxicity.
We have interconnected human gut and liver models to study organ–organ crosstalk for predicting toxicity, estimating bioavailability and modeling inflammatory disease processes.
- Allows the combined effects of intestinal and liver metabolism to be explored
- Organ–organ interaction and communication between the models can be used to compare oral and IV dosing regimes
- The open system configuration allows sampling of circulating drugs, metabolites, and biomarkers to generate concentration–time profiles
- Drug-related processes can be investigated in pharmacologically relevant concentrations
Lung/Liver-on-a-chip model
Our lungs are the main entry point into the body for a multitude of external factors – both benevolent (inhaled medications) and malevolent (pathogens or particulates). A key internal organ which is highly susceptible to damage and is required for responses to external factors is the liver, which metabolises compounds and is a key player in the acute phase response – a coordinated series of events that occur in response to infection, inflammation, or trauma.
Crosstalk between the lung and liver can be determined using the PhysioMimix lung-liver dual-organ model. The model can be used to predict drug safety, efficacy and metabolism, as well as recreate human inflammatory responses and disease.
- Monitor organ-organ communication over time to understand responses to stimuli.
- Determine the pharmacokinetics of inhaled or IV-dosed drugs – particularly uptake through, or to, either organ and drug metabolism in healthy or diseased models.
- Understand the interactions of circulating immune cells with organs and the generation of an inflammatory response to stimuli administered in the lung or liver.
- Elucidate the systemic effects of disease.
Frequently asked questions
Note that the terms organ-on-a-chip (OOC) and microphysiological system (MPS) are used synonymously throughout.
What are the key criteria and features to consider when developing a microphysiological system (MPS) / Organ-on-a-chip (OOC) model or prototype?
Prof. Linda Griffith, PhD, MIT (2024): To design any MPS/OOC model, it is crucial to first decide what questions you wish to answer using the system. Following this, find the simplest model that can be utilized to generate that answer; and design a model that is easy to use and turnkey.
A second consideration is ensuring there is routine access to the components and consumables required to run the assay. By choosing a partner with a commercially available solution, such as the PhysioMimix® OOC, you can ensure access to the components and consumables required for your experiments.
When Prof. Linda Griffith’s team at MIT first started working with the liver-chip, now commercialized and re-named the PhysioMimix Liver-on-a-chip, it met all those criteria i.e. 3D cell cultures, long-term CYP450 and other metabolic activities, immune cells, etc. It was also easy to use (an important consideration for the fast-paced and mixed-ability team), so scientists with different levels expertise could set up and carry out the experiments, with minimum training requirement, or investment in maintenance.
What are the key non-negotiable criteria for a good human relevant ADME OOC model?
Dr. Amelie Moreau, Servier (2024): To accurately assess ADME issues, we need to measure the kinetics of drug compound disappearance over time. This requires larger media volumes than those offered by most microfluidic chips. To address this, we selected the PhysioMimix OOC platform for our laboratory.
Secondly, for accurate kinetics measurement, we have to ensure there is no non-specific binding; the compound must not bind to the medium during experiments. There is a known non-specific binding issue with consumables that use polydimethylsiloxane (PDMS). So for kinetics, it’s just a no-go. Furthermore, accurate kinetic measurements require the elimination of non-specific binding, where compounds adhere to the experimental medium; i.e. MPS plates/chips. Consumables utilizing polydimethylsiloxane (PDMS) are known to exhibit this issue, making them unsuitable for kinetic studies.
The PhysioMimix Multi-chip Liver-12 plates offer a large sampling volume of up to 1 mL per chip, enabling a wide range of endpoint analyses, including -omics and microscopy. Made from inert cyclic olefin copolymer (COC), these plates are more suitable for drug evaluation studies than PDMS-based alternatives. COC’s reduced propensity to absorb small molecules minimizes non-specific binding, leading to more accurate drug response assessments.
Which are the top five dual/multi-organ models prioritized by pharma companies to successfully bring drugs to market?
Dr. Amelie Moreau, Servier (2024): We are interested in all the barrier models that would be useful for ADME studies. Our primary focus is the liver, a key organ in drug metabolism. Additionally, we are exploring multi-organ models, such as Gut/Liver-on-a-chip and combinations of the blood-brain barrier (BBB), liver, and kidney. The ultimate goal is to develop and utilize metabolically active, physiologically relevant models that integrate these organs in different combination to simulate complex drug interactions.
What advice would you give someone starting out with OOC/ MPS?
Dr. Marc Beal, Health Canada (2024): Firstly, focus on optimization to ensure you answer the right questions. For us, it was crucial to know when our mouse liver-on-a-chip model was fully proliferating and most metabolically active for genotoxicity assessment. We also needed to determine DNA and RNA yields for mutation and transcriptome analysis.
Secondly, set aside a few plates for optimization. With the PhysioMimix OOC Systems, after optimization for your experiment, it’s pretty much plug-and-play. We spent more time on mouse liver perfusion than on the actual MPS studies – they’ve been very easy to do.
Do you have any advice for new OOC/MPS users, especially those working with a multi-organ system?
Dr. Amanda Clark, University of Pittsburgh (2024): When working with a multi-organ system, it’s crucial to consider the matching of cell types and donors. For our liver-chip, we use both primary hepatocytes and donor-matched non-parenchymal cells. This is important because the nonparenchymal cells significantly impact the behaviour and fate of cancer cells. For our gut model, we use stem cells that are not donor matched. However, as long as we use “somewhat” primary human cells in both models, they answer our research questions.
Since our focus is on cancer, we avoid using cancer or immortalized cell lines. It’s essential to use primary cells (or cells as close to primary as possible) in a human setup. This allows us to accurately dissect species-specific signaling and interactions that occur within humans, which differ from those in mice.
What was your experience of building a mouse liver-on-a-chip and comparing it to human models?
Dr. Marc Beal, Health Canada (2024): In our research division, we have decades of experience with animal research on specific hepatotoxins, which provides a solid foundation for validating new methods. We aim to determine whether the liver-on-a-chip model behaves more like an animal model or a 2D culture environment, especially when dealing with chemicals that elicit different responses in humans and mice.
For instance, aflatoxin B1 is a potent human hepatic carcinogen but has minimal effects on mice. When we shift our focus to using a human-relevant model, we want to ensure that any differences we observe are due to biological factors rather than technological discrepancies. This approach helps us accurately reflect human biology. Currently, we are developing our mouse liver-chip and will soon be working more closely with HepaRG cells to further our research.
Considering the 3Rs as a driver, how do you see the future need for cross-species assays and human Organ-on-a-chip models
Annie Hamel, Charles River Laboratories (2024): The FDA want to see evidence that MPS models provide results that are comparable to animal models, and those that can support replacing animal testing. At Charles River Laboratories, we also aim to show that MPS models yield results more aligned with human outcomes.
How do OOC technologies compare to organoids as a replacement for animal models?
Dr. Tomasz Kostrzewski: Organoids and OOC share a lot of similarities. Both models include multiple cell types that produce tissue-level functionality, but organoids are comprised of a few thousand cells, whereas cell numbers in an OOC could be in the millions. This greater density of tissue facilitates metabolic capacity and biomarker detection, which is not be possible, when dealing with the smaller cell quantities of organoids.
Many organoid cultures demonstrate high levels of functionality, equivalents to primary human ex vivo tissues. OOC functionality, similarly, is dependent on the source cells used in the culture, and how they compare to native tissues.
Finally, organoids can also be used to grow OOC cultures. A numbers of users have taken stem-cells-derived organoids to seed OOCs, to bring the advantage of perfusion, peripheral immune cells, and the capability to link multiple organs together to form complex multi-organ-on-a-chip model.
Can you have mixed organs on a single chip to integrate if they have collaborative function as we see among organs in the whole animal?
Dr. Tomasz Kostrzewski: Numerous different Organ-on-a-chip providers have successfully produced multi-organ model. We were lucky to be part of a DARPA funded consortium to build a body-on-a-chip a few years ago, where we successfully connected 10 individual organs. However, none of the OOC providers are quite ready to offer a commercial body-on-a-chip solutions, even today, but several organizations are making strides in producing functional multiorgan models.
Interesting links can be investigated using multi organ microphysiological systems (MPS). For example, a Liveron-a-chip can be linked to a Pancreas-on-a-chip to look at insulin resistance and metabolism, we can investigate the crosstalk between organs, where inflammatory responses play a role. At CN Bio, we have partnered with Altis Biosystems to offer our customers a Gut/Liver-on-a-chip model to assess ADME properties / oral bioavailability. This assay has been developed into our PhysioMimix Bioavailability assay kit: Human 18 and can also be accessed through our Contract Research Services.
Commercial entities, including CFI, also have generated multiorgan-on-a-chip solutions. A whole range of different modular problems can be addressed with multi-organ MPS technology. While their availability remains less abundant than single-organ models, and they are yet to be broadly validated and adopted, multi-organ MPS can offer valuable insight in particular contexts of use.
Dr. Clive Roper, Roper Toxicology Consulting: Often, we expect MPS models to provide answers to numerous questions at the same time, but if we carefully define the contexts of use, we can address different parts of a mechanism with different test systems. So, we don’t have to develop a single system that offers all the answers but design experiments in a way that ensures we pair the right question with the right solutions. We might need multiple steps to reach the overall conclusions. We’re increasingly accepting that a single experiment will not directly replace an animal model, but using a set of multiple components can address the same questions adequately.
If you do not find the answer to your question listed, please contact us