Speak to an expert about 3D validated cells
PhysioMimix® consumables
3D validated cells
Accelerate organ-on-a-chip research with 3D validated cells

Expedite the delivery of human translatable data by eliminating the burden of cell validation.
Pre-validating cells for organ-on-a-chip (OOC) use is frequently overlooked, yet critical to assay success. Use of unvalidated cells results in failed microtissue formation, poor assay performance and unreliable data. The validation process is necessary, but it is also costly and time-consuming.
Sourcing two validated hepatocyte lots for OOC assays costs us around $15K in materials and 100+ hours of a trained scientist’s time.
Our 3D validated cells portfolio eliminates this burden from your laboratory workflow! Choose from our catalog to fast-track the recreation of PhysioMimix OOC models.
3D validated cell features
- A broad number of individual donors are available
- High availability of vials per donor
- Proven metabolic activity, tissue functionality, and 3D growth
- Guaranteed for culture up to two weeks, maintaining function and phenotype
- Validated for mono and co-culture assays (see applications section)
- Tested for use with PhysioMimix OOC Systems and SOPs
Benefits of using 3D validated cells

Eases your transition into OOC by using cells that are proven to thrive

Eliminates the burden of in-house quality control testing with convenient, off-the-shelf access

Delivers reproducible inter-assay performance for data confidence

Focuses your time on value-add experiments

Cells sourced from LifeNetHealth LifeSciences or Lonza
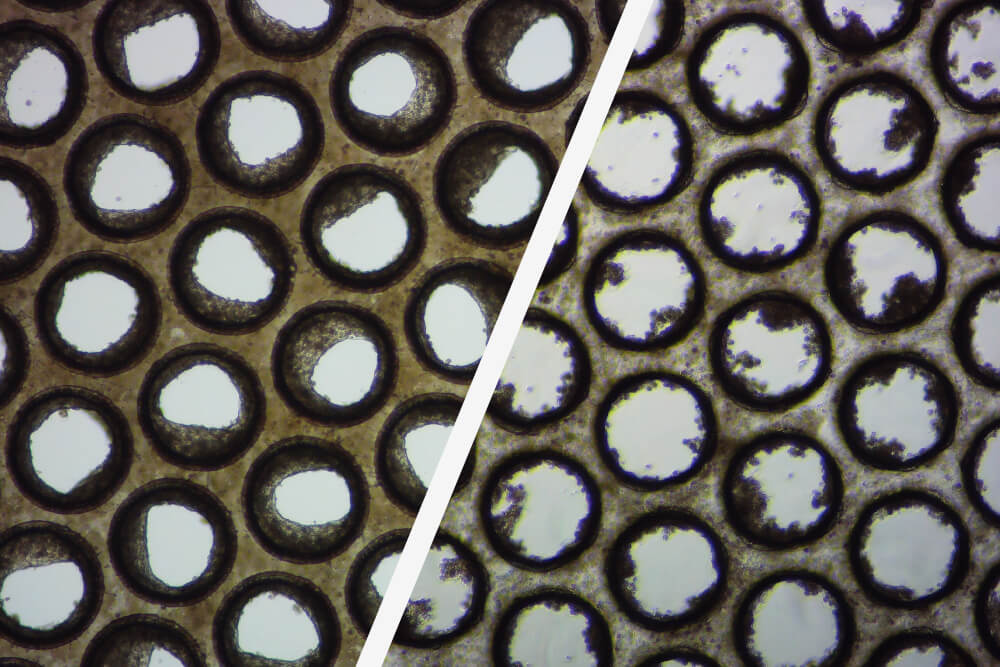
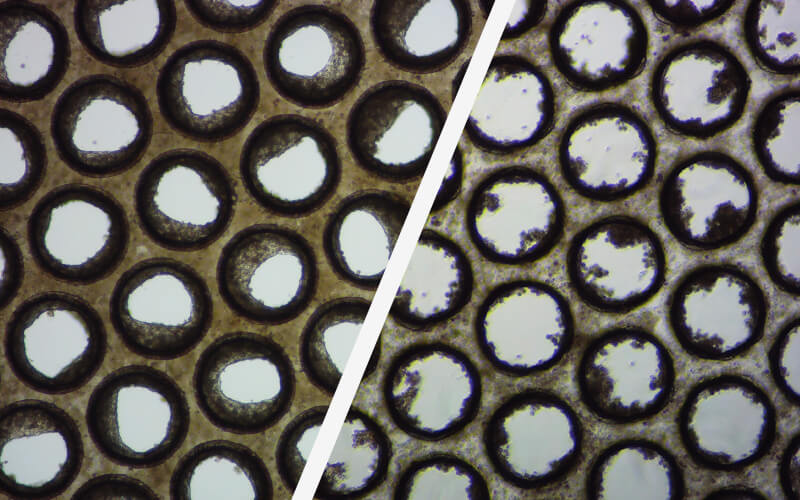
good / poor liver microtissue
Currently available 3D validated cells*
| Source | Cells/vial | Order from | |
| Primary human hepatocyte cells | LifeNet Health LifeSciences | min 5M/vial | LifeNet Health LifeSciences |
| Primary human Kupffer cells | LifeNet Health LifeSciences | min 500K/vial | LifeNet Health LifeSciences |
| Primary human hepatocyte cells | Lonza | min 5M/vial | CN Bio |
| Primary human Kupffer cells | Lonza | min 500K/vial | CN Bio |
*Some geographical regions may have legislation for storing human cells requiring a license
Hepatocyte thawing medium
Hepatocyte thawing medium is not supplied. To maximize post-thaw hepatocyte yield, it is imperative that you purchase hepatocyte thawing medium from the relevant manufacturer.
The effect of poor lot quality
>60% of the primary human hepatocyte lots tested by our experts in the last five years have failed to form liver microtissues or meet acceptable performance limits in OOC assays.
These brightfield images demonstrate the formation of good (left) and poor (right) liver microtissue formation within the pores of individual Multi-chip Liver-12 plate scaffolds.
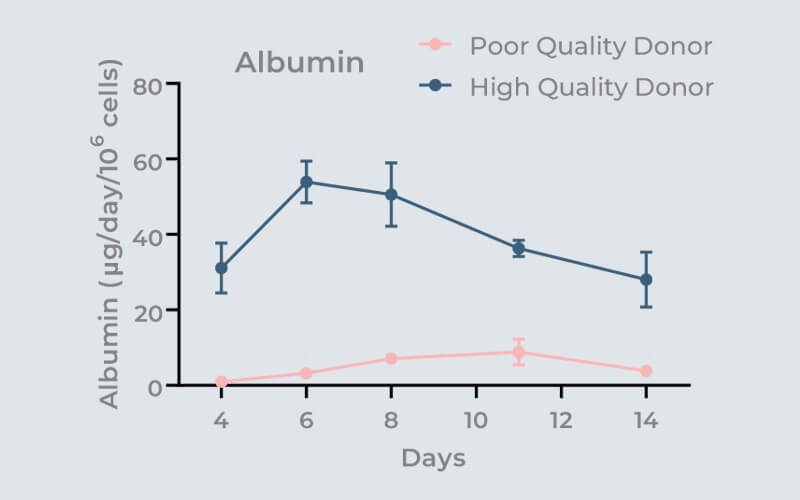
Highlighted in the graph is the effect of donor quality on microtissue functionality (albumin production) over 14 days. Eliminate the risk of poor assay performance by using 3D validated cells.
Validation reports
Our sample reports describe the extensive process of validating donor cells for use in OOC assays. Our quality control procedures ensure that mono-or co-cultures form highly functional, stable three-dimensional liver microtissues.
Supported applications
To serve a range of different application needs, 3D validated cells can be purchased for mono-or duo-cultures. Tri-cultures are available within NASH-in-a-box kits.
| Liver model | Cell type | Applications | Required products |
| Mono-culture | Primary human hepatocytes | HBV, Drug metabolism | PhysioMimix OOC, Liver plates, 3D validated cells |
| Duo-culture | Primary human hepatocytes + Kupffer Cells | HBV, DILI, Immune-mediated toxicity | PhysioMimix OOC, Liver plates, 3D validated cells |
| Tri-culture | Primary human hepatocytes + Kupffer Cells + Stellate Cells | NASH | PhysioMimix OOC, NASH-in-a-box |
Frequently asked questions
Do you supply Safety Data Sheets?
Once ordered, cells are drop-shipped to you directly from the source supplier. Please contact LifeNetHeath LifeSciences or Lonza directly to request any associated materials that are not supplied within the shipment.
How do I order 3D validated cells?
3D validated cells from LifeNet Heath LifeSciences can be ordered directly from LifeNet Heath LifeSciences. Contact us for information about lots that have been validated for use with PhysioMimix OOC Systems.
3D validated Lonza cells must be ordered through CN Bio directly.
Are shipping costs included?
Shipping costs are not included as they may vary depending on the number of vials ordered or split shipment requests. Shipping costs will be added as a line item in any quote requests.
Why did you choose to work with LifeNet Heath LifeSciences and Lonza over other vendors?
LifeNet Health LifeSciences is an innovative leader, trusted collaborator, and reliable solutions provider – with a commitment to providing game-changing innovations in human in vitro biology. They offer the largest selection of comprehensively characterized liver cells and tissue in the industry, including healthy and diseased donor-matched samples. The organization is the only biospecimens provider in the world that spans the donation continuum – from ethical donor consent to protocol-based recovery by highly trained personnel, to tightly controlled transport logistics with minimal ischemia time. Additionally, LifeNet Health LifeSciences also offers a high level of technical support. More information about their commitment to quality can be found here. Discover more about the company at LNHLifeSciences.org. View our joint press release here.
Lonza hepatocyte cells meet the high standard of quality required by organ-on-a-chip systems. With a robust manufacturing process and excellent quality control, state-of-the-art facilities and Lonza’s decades of specialist experience and deep expertise, Lonza cells offer a greater chance of reproducible success. Access to a wide variety of large-sized lots gives customers confidence in ongoing cell availability. View our joint press release here.
Who should I contact with my enquiries?
For anything other than data sheet requests please contact us directly to ensure a streamlined response. Where necessary we will reach out to the responsible supplier.
How should 3D validated cells be stored?
Prior to use, cells should be stored in the vapor phase of liquid nitrogen.
I don’t see the cell type(s) I am interested in within your 3D validated cells catalog
Our development roadmap includes the qualification of additional cell types. When ready these will be added to our catalog of 3D validated cells to facilitate the recreation of other single and multi-organ models in our portfolio. Until available, we can provide you with recommendations based on our experience and/or guidance to validate your own cells prior to their use in OOC assays.
If you are developing your own OOC model, cells are the most critical parameter to consider, validate and quality control. Regardless of the model you are developing, various technical factors should be considered to validate cells for OOC assays. Here are the most important considerations.
- Decide what type of cells (primary versus iPSC versus immortalized etc.) you want to use
- Check the number of accessible vials from the vendor to ensure supply
- Identify the number of cells per vial
- Test for cell viability (over 80%)
- How well do they perform in mono- and coculture with other cell types?
- Do they form 3D tissues with the correct pathophysiology?
- Are they functional (secreting any functional soluble biomarkers)?
- Do they behave the way the target organ behaves?
- Any there clinically relevant endpoints you can use to translate data to the human?
If you do not find the answer to your question listed, please contact us