Blogs

6 Challenges in ADME Drug Development
Explore the current challenges in profiling preclinical human ADME through key publications. Can advances in the human relevance of preclinical models improve estimations for safer and more efficient drug development?
Webinars

Organ-on-a-Chip: Big Questions Surrounding Regulation and the Roadmap to Broad Adoption
To enhance and modernize the drug development process, many organizations are adopting Organ-on-a-chip (OOC) technologies into their workflows, from efficacy to safety assessment. With new developments in the technology and its applications evolve, questions and opportunities are arising, especially with regards to the regulatory landscape for NAMs , cost efficiency, and model confidence through standardization.
Scientific publications

Liver-on-chip model and application in predictive genotoxicity and mutagenicity of drugs
Kopp et al., 2024. Current pre-clinical drug safety assessments lack a single, comprehensive test system for genotoxicity hazards identification. This study, aimed to develop an in vitro model to addresses this gap, looks at Organ-on-a-chip (OOC) technology as a solution. OOC present robust human metabolic activity and has the potential to assess all required endpoints for genotoxicity hazards identification, ultimately streamlining the process and eliminating the need for animal testing. This proof-of-concept experiment demonstrates the potential of PhysioMimix® Liver-on-a-chip model as a promising tool for in vitro genotoxicity hazards identification, paving the way for a more streamlined and animal-free pre-clinical drug safety assessment process.
Posters

Defining validation criteria for a primary jejunum and primary hepatocyte dual-organ MPS
Improve in vitro to in vivo data translation for drug safety and efficacy with our fluidically-linked dual-organ MPS (microphysiological system); combining two well-characterized human Gut/Liver MPS – the RepliGut® Jejunum and PhysioMimix® Liver MPS, in an interconnected model suitable for enhanced bioavailability profiling.
Blogs

How in vitro human Gut/Liver models increase confidence in ADME estimations before human trials
This blog explores a world where novel multi-organ in vitro models, tiny replicas of the human intestine and liver, provide the key to unlocking a deeper understanding of how we Absorb, Distribute and Metabolize drugs.
Read more to discover why accurately estimating human ADME remains a challenge and a potential solution that enables greater confidence in lead candidates and a reduced risk of identifying poor oral bioavailability during first in human studies.
Blogs

Organ-on-a-chip adoption: The roadmap to broader use
This blog explores challenges faced by the Pharmaceutical industry that pave the way for Organ-on-a-chip adoption and why it makes sense to start with toxicology workflows.
Posters
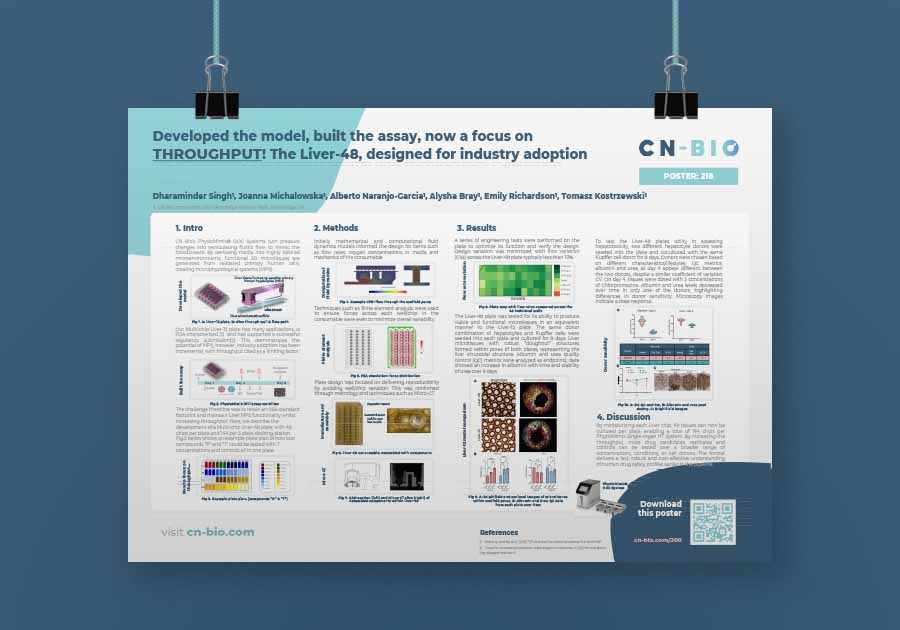
Developed the model, built the assay, now a focus on THROUGHPUT! The Liver-48, designed for industry adoption
The PhysioMimix® Liver-48 plate poster: CN Bio’s Multi-chip Liver-48 plate retains SBS-standard footprint, maintains Liver MPS functionality, and increasing throughput. By miniaturizing each chip, 48 tissues can be cultured per plate, 144 chips per PhysioMimix Single-organ HT System – more replicates and controls for fast, robust and cost-effective understanding of human drug safety earlier in the pipeline.
Blogs

How Organ-on-a-Chip Addresses R&D Challenges
A blog that explores how organ-on-a-chip models are transforming liver disease research.
Application notes

Developing a translational biomarker panel for use in pre-clinical advanced in vitro studies of Non-alcoholic steatohepatitis
A pressing need for more human relevant pre-clinical tools to support therapeutic advancement has led to the development of Microphysiological systems (MPS) that more closely model the human liver and NASH.
Blogs

Understanding the mechanism of toxicity: OOC’s crucial role
It’s commonly acknowledged that the current drug discovery process is inefficient with large numbers of drugs failing in the clinic. Almost a third fail due to undetected toxicity issues. Countless more potential drugs fail due to misclassification by animal models.
Application notes

Connecting the gut and liver
A human-relevant dual-organ microphysiological system connecting the gut and liver for preclinical profiling of oral bioavailability
Efforts to improve the in vitro to in vivo translation of drug efficacy and safety data has led to the emergence of more human relevant microphysiological systems (MPS). Multiple, fluidically linked MPS can be linked to form multi-organ systems that simulate human processes which can be utilized to improve ADME and bioavailability estimations. ADME and bioavailability are central in determining the safety and toxicology profiles of compounds and are therefore crucial preclinical drug development measurements.
Posters

Connecting the human intestine and liver
A primary jejunum and primary hepatocyte multi-organ MPS for more predictive studies of human drug ADME and oral bioavailability
Traditional immortalized intestinal cell lines and suspension hepatocytes have absent or low levels of metabolic enzyme expression, and thus fail to predict first pass human metabolism and oral bioavailability.
Efforts to improve the in vitro to in vivo translation of drug efficacy and safety data has led to the emergence of more human relevant microphysiological systems (MPS) that consist of multiple, fluidically linked organs1.

