Liver-on-a-chip models
In vitro Liver-on-a-chip models with unrivaled human relevance
Our predictive human Liver-on-a-chip (or LiverChip), models are cultured in Multi-chip Liver plates by the PhysioMimix® OOC range of microphysiological systems.
They recreate the multi-cellular architecture of the liver, enabling drug metabolism and transport, drug-induced liver injury and chronic liver disease studies.
Getting started is easy. We’ve made the complex simple by supplying fully validated SOPs, 3D validated cells and “in-a-box” complete solution kits.
Why use our Liver-on-a-chip models?
Multi-cell co-cultures
Combine hepatic and immune cell types to recreate the liver microenvironment
3D microtissues
Hepatocytes adhere to create highly polarised functional microtissues supported by scaffolds
Fluidic flow
Provides essential nutrients, O2 and biomechanical stimuli to promote culture longevity
Physiological cues
Accurately model a variety of diseases such as NAFLD/NASH, Hepatitis B and liver cancer
High metabolic activity
Permits human phase I and II metabolism, drug transport, and metabolite-induced toxicity studies
Flexible cell compatibility
Primary cells, immortalised cell lines, stem-cell or patient-derived tissue slices

Measurement of clinical biomarkers
Enables a smooth transition of data between the laboratory and the clinic
High inter- and intra-plate reproducibility
High inter- and intra-plate reproducibility delivers robust and reliable data
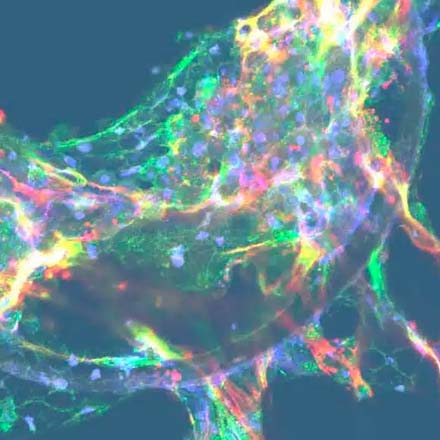
Dynamic 3D microenvironment
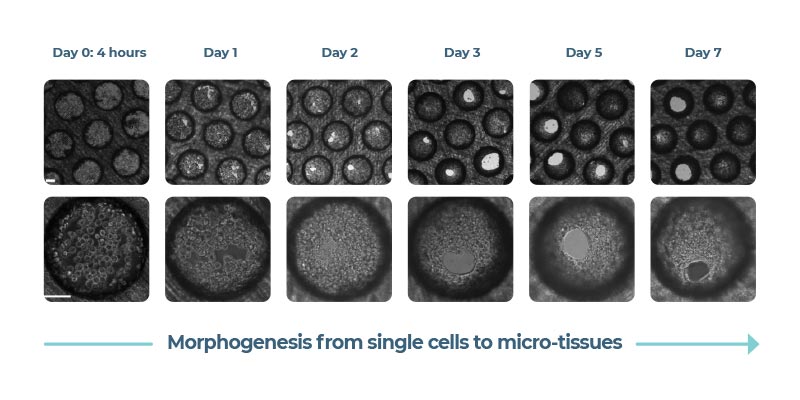
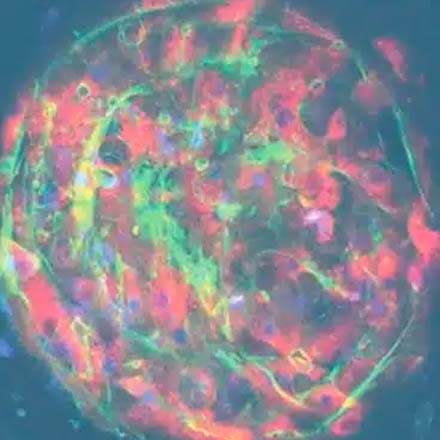
Primary hepatocytes and non-parenchymal cells are cultured to form 3D microtissues in bespoke engineered scaffolds. Microtissues are continuously perfused by media to mimic blood flow.
The dynamic 3D microenvironment of the Liver-on-a-chip promotes the viability and functionality of liver cells, enabling their long-term culture for up to four weeks.
Clinical translatability
Using Liver-on-a-chip models, multiple clinically relevant biomarkers can be monitored over time from single samples. The model expresses human-relevant levels of phase I/II enzymes and transporters.
They permit an almost limitless number of end points to be profiled from acute and chronic studies and improve the predictive performance of in vitro assays by delivering data proven to translate into clinical outcomes.
Ultimate flexibility
Work the way you want to with the PhysioMimix suite of hardware, protocols and consumables.
- Application-based SOPs and primary human 3D validated cells support the easy re-creation of our industry validated mono- and duo-culture models.
- Our NASH-in-a-box kit provides an off-the-shelf solution to fast-track the adoption of our human Non-alcoholic steatohepatitis model in your laboratory
- Adapt the model to match your research needs. Incorporate physiological combinations of additional cell types such as non-parenchymal cells (NPCs), immune cells (innate/adaptive), or exogenous stimuli, as required.
Fully validated
Liver-on-a-chip models
| Liver model | Cell type | Applications | Required products |
|---|---|---|---|
| Mono-culture | Primary human hepatocytes | HBV, Drug metabolism | PhysioMimix OOC, Liver plates, 3D validated cells |
| Duo-culture | Primary human hepatocytes + Kupffer Cells | HBV, DILI, Immune-mediated toxicity | PhysioMimix OOC, Liver plates, 3D validated cells |
| Tri-culture | Primary human hepatocytes + Kupffer Cells + Stellate Cells | NASH | PhysioMimix OOC, NASH-in-a-box |
Liver-on-a-chip applications
Disease modeling
Safety toxicology
ADME
Frequently asked questions
Please describe how the 3D structure of the Liver-on-a-chip model is generated?
Liver-on-a-chip models, also known as Liver Chips, or Liver microphysiological systems (MPS) are cultured in either Multi-chip Liver-12 (12 chips or wells/plate), or Liver-48 (48-chips or wells/plate) plates by PhysioMimix OOC Systems. Each well contains an individual, enclosed recirculating perfusion system and a bespoke collagen-coated scaffold containing microchannels, or pores, in which primary liver cells are seeded. For each plate type, the dimensions of the scaffold’s microchannel remain the same, however, there are ~75% fewer microchannels/scaffold in the Liver-48 plate versus the Liver-12 plate. This permits the model to be easily miniaturized enabling higher throughput without loss of function.
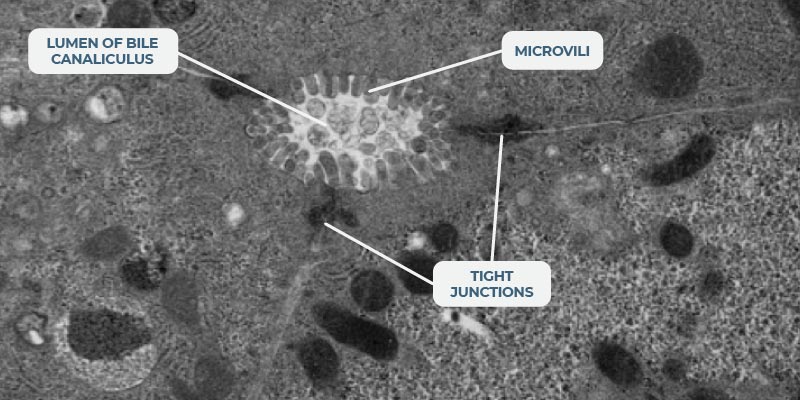
Our bespoke scaffolds a) promote the three-dimensional (3D) formation of a liver microtissue and b) ensure that the maximum surface area is exposed to flowing media. The system’s microfluidics mimic human blood flow to the liver. A circular and unidirectional flow pushes the cells within the scaffold’s microchannels. Some liver cells attach to the microchannel walls, while others attach to their neighboring cells, thus creating a network of liver tissue. Fluidic flow provides nutrients, oxygen, and biomechanical stimuli to all the cells within the 3D structure to promote microtissue formation and ensure culture longevity. 3D tissues are polarized and feature key liver structures, such as bile canaliculi and their microvilli, to recapitulate the liver’s microarchitecture.
Please view this animation for more information.
What is the physiologically relevant flow rate for liver? Should the liver experience any meaningful shear?
In the liver, hepatocytes experience very low (near zero) shear stress as they are separated from the blood by the fenestrated endothelium. However, as the endothelium is fenestrated (i.e. has holes), there will be substantial mass transport, and this will be influenced by the flow rate in the sinusoid. Our PhysioMimix OOC Systems provide sufficient flow to ensure good mass transport with low shear stresses of a similar order of magnitude to those observed in the liver sinusoid/capillary. To learn more about the flow in our system please read: Domansky et al, 2010 and Ebrahimkhani et al, 2014.
Do we have any control over this flow rate?
We provide optimized and recommended flow rates that are in line with human physiology, even down to the type of flow – pulsatile, or continuous. However, flow rate is a customizable feature. If you are particularly concerned about the sensitivity of your cells, just use the software on the controller to lower the flow rate (as low as 0.5 µL/second). Conversely, you can increase it (up to 2.5 µL/second) should you want to. The maximum rate achievable is still within healthy liver range.
Have you assessed the effect of shear stress on hepatocytes directly exposed to flow, vs the ones located “inside” the 3D structure?
By adapting the scaffold design and system microfluidics, we explored different microenvironments within the Multi-chip Liver-12 plate to ascertain the levels of shear stress and forces, flow rate, oxygen gradients and more within our liver cultures. We utilized a combination of experimental data and computational fluid dynamics to determine the most physiologically relevant settings, which in turn led to improved cell morphology and functionality for up to 4 weeks (Rowe et al. 2018). For more information, please watch this on-demand webinar: https://webinars.cn-bio.com/the-rhythm-of-life.
How uniform is the liver microtissue formation across the microchannels/pores?
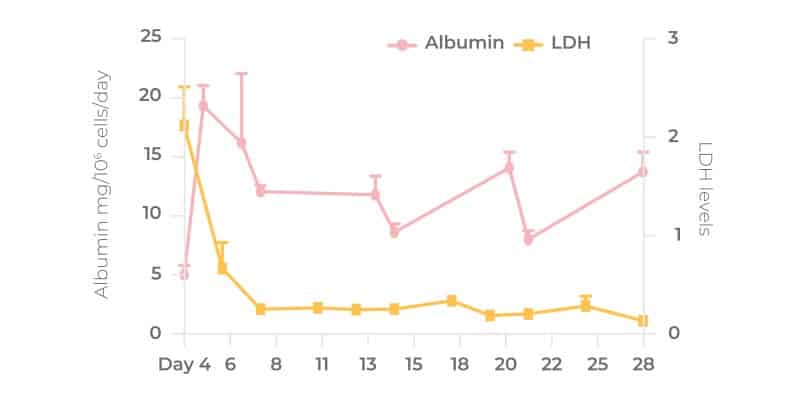
The microtissues form by self-directed assembly from a pre-mix of cells added to the scaffold and are relatively uniform across the pores. There are approximately 2,000 cells per pore. Note, not all primary cells will form highly functional 3D microtissues, therefore it is important to pre-validate cells ahead of time. Users can perform QC checks by testing different donor lots, or they can circumvent this step to save valuable, resource budget and time by purchasing 3D-validated cells from us directly. Albumin expression is used as a biomarker for cell health throughout the experimental timeline and has been shown by our R&D team to remain robust for upwards of 28 days. Microtissue activity is measured by sampling media from each well (not per pore) so there is no requirement for normalization.
How many primary human hepatocytes are seeded in one Liver-on-a-chip experiment?
Using a Multi-chip Liver-12 plate, PhysioMimix OOC Systems enable you to run up to 4 conditions in triplicate per plate, with a well-defined and optimized seeding number of 400,00-600,000 Primary Human Hepatocytes (PHH) per well either in mono- or co-culture with non-parenchymal cells.
This number of cells enables the generation of truly high content data. Various endpoint analysis, such as -omics and microscopy, and clinical endpoints can be performed at the same time on one sample. Please visit the relevant application pages for a list of common output measurements.
Using a Multi-chip Liver-48 plate, PhysioMimix OOC Systems enable you to run up to 16 conditions in triplicate per plate, with a well-defined and optimized seeding number of 150,000 PHH per well.
How long do liver-on-a-chip microtissues stay alive in your Liver-on-a-chip?
Liver-on-a-chip microtissues are viable for over 28 days without loss of cell function or dedifferentiation. We have published data (Ortega-Prieto et al, 2018) showing viable functional cultures up to 40 days for the Liver-12 plate, so the system could potentially be used for longer, however, we tend to run assays between 8-14 days depending on the application. For certain applications – examples being the analysis of low clearance compounds and the induction of chronic liver disease pathophysiology, culture longevity provides huge benefits.
Do liver-on-a-chip microtissues develop bile canaliculi?
Although we have not explored microtissue architecture to a great extent, we have stained markers that are trafficked into the canaliculi space, and we have also labelled with antibodies for the expression of markers on the canaliculi. When we look at scanning electron microscopy (SEM) of the actual liver-on-a-chip tissues you can see lumens, with microvilli formation, developing for the bile (please refer to the image in the “Dynamic 3D microenvironment” section of this page).
How do you confirm the presence of different cell types in your organ-on-a-chip models?
When developing any coculture model, confirming the presence of the required cell is critical. Depending on the organ that you are trying to recapitulate and its composite cell types, specific biomarkers can be used. In our duo, and tri-culture liver models, for example, Kupffer cells are incorporated to represent the liver’s innate immune system. Their presence is confirmed by measuring inflammation levels, via inflammation biomarkers (e.g., interleukins or cytokines) versus control primary human hepatocytes monocultures (PHH). In our triculture model of Non-alcoholic steatohepatitis (NASH), stellate cells are also added, and their presence confirmed by comparing fibrosis levels to PHH monocultures in a fat media (Kostrzewski et al 2021). Transcriptomic analysis to detect the RNA expression of cell-specific markers provides an alternative approach.
Can you incorporate more liver cell types into your liver-on-a-chip model?
The short answer is yes, it is possible to customize the type of cells used. The architecture of our Multi-chip plates is open, rather than closed. This makes it easy to customize your OOC models to make them your own by adding more cell types in. However, the decision to add more cells in really depends on what you are trying to achieve, plus it will increase the cost of your experiment. When developing advanced in vitro models, we assess which cell types are required for each application. For example, our standard healthy liver model, mainly used for drug metabolism studies, is comprised of a monoculture of primary human hepatocytes (PHHs). However, for studies requiring inflammation or other hepatic features, we will add non-parenchymal cells (NPCs) such as Kupffer, stellate cells, or recirculating immune cells. Our models can, and have been, further adapted by our customers (published in a poster at SOT in 2022) to replicate the liver endothelial fenestrae by including Liver sinusoidal endothelial cells (LSECs)
Have you tried other liver cells such as induced-Pluripotent Stem Cells (iPSCs)?
We have indeed used iPS- hepatocytes in our liver-on-a-chip to show proof of concept, but we have not fully validated the models. In the main, we work with primary cells whenever possible because of their human relevance. We have demonstrated that the iPSC-Hepatocytes (iPSC-Hep) form a similar microtissue to our primary human hepatocyte (PHH) model. The tissue offers improved hepatic function and metabolic activity compared to standard 2D iPSC-Hep models, but in our hands, the functionality of PHH’s (cytochrome p450 activity) is higher than iPSC-derived hepatocytes. Primary cells also remain in a differentiated state for longer (a month) in culture when perfused to replicate blood circulation.
There are pro’s and con’s for both cell types, which we explore in a bit more detail in this recent blog. In jurisdictions in which the use of human tissues is restricted, stem cells represent the obvious alternative. Primary cells also have limitations that can be addressed by iPSCs e.g., accessing a certain phenotype, or multiple cell types from the same donor. So, although iPSCs lack the genomic stability of primary cells and are prone to spontaneous differentiation into other cell types, they offer greater “flexibility” as they can be reprogramed into the right tissue model, with a specific phenotype. It should be noted that our own limited experience of working with iPSCs limits the level of support that we can provide for iPSC-derived Liver-on-a-chip models.
Can you develop animal Liver-on-a-chip models?
This has been a growing area of interest over the last couple of years and one we are actively developing. We can culture beagle, rat, and other animal hepatocytes in our PhysioMimix® OOC Systems and Multi-chip Liver plates. While the ultimate use of these systems is defined by the customer, this approach provides a means to assess the translatability of data between in vivo animal models and a Liver-on-a-chip version of the animal model and, by default, greater confidence in data translatability between human organs and their OOC counterparts.
How different is your 3D liver-on-a-chip model to 3D spheroid models?
Culturing primary human hepatocytes (PHH) as spheroidal structures, generated by gravitational aggregation in hanging-drops or on ultralow attachment surfaces, offers a high-throughput method for assessing drug responses. A wide variety of models have been developed to include plated micro-patterned co-cultures of hepatocytes with stromal fibroblasts, 3D bio-printed liver tissues and 3D spheroid cultures with or without hepatic non-parenchymal cells (NPCs). However, all these methods still have drawbacks such as a short lifespan, loss of phenotype over time (dedifferentiation) and low metabolic activity. Traditional in vitro PHH models lose key hepatic functions, such as metabolic activity, during short-term culture. Comparatively, the lifespan and functionality of PHHs co-cultured in a more physiologically relevant perfused microenvironment (using the PhysioMimix OOC) are significantly extended. This enables prolonged “chronic” drug exposures to be investigated and more complex biological interactions to be studied (Long et al., 2016).
A recent blog explores how organ-on-a-chip models are taking organoids to the next level.
How does the Liver-on-a-chip model perform compared to other in vitro alternatives?
While simpler in vitro systems successfully flag yes/no drug responses, these are perfectly complimented by the use of more advanced perfused OOCs to provide the detail. Achieving data richness and high assay sensitivity requires volume. In the Liver-12 plate, approximately half a million primary cells perfused by large volumes of media (>1 mL) generate enough horsepower to reproduce phase 1 and 2 metabolism (particularly important because one of the reasons that standard animal and in vitro models have proven to be poor predictors due to their non-human metabolic profiles) and to increase assay sensitivity such that more sophisticated clinical chemistry outputs of liver function such as ALT/AST can be detected.
Samples taken over time in longitudinal studies provide biochemical assessments that build up a picture over time. The relatively large amount of tissue recovered at the end of the experiment can be examined in additional proteomics or genomics studies to look at gene up- and down-regulation to inform a more complete mechanistic profile of drug action.
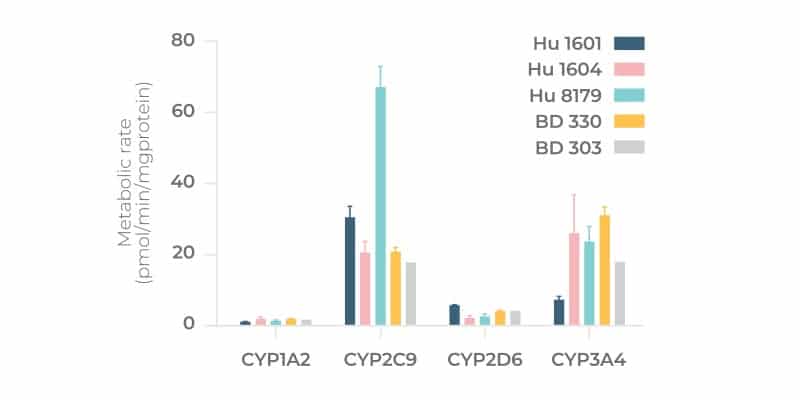
Hepatocyte performance in PhysioMimix OOC Systems is significantly improved compared to other standard approaches such as collagen-sandwich cultures and spheroids. CYP activity can be detected throughout the culture period as can the expression of transporters. For an independently generated comparison please see our peer reviewed co-publication with the US FDA
Read the following blog to find out more.
Is the establishment of hepatic zonation something you have looked into?
We have previously demonstrated that there is an oxygen gradient across the scaffolds within our Multi-Chip Liver-12 plates, therefore the cells across the scaffold will experience slightly different oxygen concentrations (Domansky et al., 2010). The scaffold, however, is 250 μm thick so its depth is not enough to mimic hepatic zonation. The system can be run under different oxygen concentrations in the cell culture incubator to allow different areas of the liver to be modeled.
Can you recover liver-on-a-chip microtissues to perform transcriptomic profiling?
Yes, one of the advantages of our approach is that large amounts of recoverable tissue are available at the end of the experiment for endpoint analysis, including transcriptomics and other ‘omics-based techniques. Several publications demonstrate this including e.g. Vacca et al. 2020 and Kostrzewski et al 2021.
Do you measure transporter expression or function in your Liver-on-a-chip models?
We can measure both transporter expression and functionality for a variety of transporters. Expression can be measures using qPCR or fluorescence microscopy methods, whilst the use of transporter inhibitors enables testing of transporter functionality.
How do you account for donor-donor variability in Liver-on-a-chip models?
That is an interesting question as this has always been a challenge to perform in vitro. There are several things to consider when donor-donor variability is considered:
- What is the adequate number of donors to include in a study, without drastically increasing the cost and timeline of the study?
- What genetic, gender or ethnic background should I look for in the different donors?
- Can I access a diverse type of donors and source good donors that perform well under my experimental conditions?
When validating donors, we always try to find the right compromise between the requirement for different donors and the use of high-quality donors. The number of different donors that you can access greatly depends on cell supplier availability, which is why it is important to source from more than one supplier. As previously mentioned, the number of donors that you evaluate within a study affect the cost of the study. The rule is simple, the more donors you incorporate, the more expensive the study will be. One way to minimize cost is to use pooled donors, which are a good compromise for rapid assessments. However, in our experience, pre-mixed pooled donors purchased off-the-shelf generate poor quality microtissues in OOC culture. If you want to run a study using pooled donors, we recommend identifying three to five donors that first pass pre-validation tests individually (to test for liver microtissue formation and function) before pooling together (Tsamandouras et al, 2017). See this recent blog for more info about 3D validating cells, or circumvent this step by sourcing 3D validated cells from us directly.
As practical as pooled donors are, they do also have limitations. For example, one donor may be more represented than the others in the microtissue as there is no control over which donor cells attach best to form tissue. Using a higher throughput OOC system for these studies can help reduce the financial and resource burden as more conditions can be combined into one Multi-chip Liver plate. For example, our PhysioMimix Single-organ HT System and its Liver-48 plate enables four times (n= 16 conditions in triplicate) the experimental capacity of our standard PhysioMimix Liver-12 plate.
This recent poster from the US FDA “Characterization of the effect of primary human hepatocyte (PHH) lots on function in CN Bio Innovations Liver-Chip using acetaminophen” provides some useful insights into the effect of donor-to-donor variability in OOC assays.
If you do not find the answer to your question listed, please contact us