Mirror, mirror on the wall, which is the best cell of them all?
If you are considering incorporating Organ-on-a-chip (OOC) technology into your workflow, you or your team may not know quite where to start.
Using a commercially available solution, such as our PhysioMimix® OOC, makes the transition from 2D, or simpler 3D organoid/spheroid assays, straightforward. If you are at this stage, you may find our infographic “Top tips for integrating organ-on-a-chip technology into your workflow” useful.
For those who have already chosen a preferred solution, your next consideration is to ensure that the data you generate is meaningful. This requires 5P’s…
- Prior
- Planning and
- Pre-validation to ensure
- Perfect assay
- Performance.
During this phase, we cannot emphasize enough the importance of choosing the right cells. Not all cells perform well in OOC assays and not all cell types offer the highest degree of human relevance! This in-depth blog explores the steps that we go through during our experimental planning phase to pre-validate cells for use in OOC assays. Read on to identify the easiest way to invest in your success – choose 3D validated primary cells from our portfolio or, how to embark on your own pre-validation studies.
Why use primary human cells over other cell types in OOC assays?
One of the biggest topics of debate within the scientific community is the choice of cell type, primary versus cell lines, or induced pluripotent stem cells (iPSCs). On one hand, engineered cell lines are simple to handle, readily available and cost-effective plus, they deliver reliable and precise data. Combined, these factors mean that engineered cell lines are ideal for high throughput screening settings. On the other hand, they frequently lack key cellular components and have cancer-like characteristics and therefore fail to adequately recapitulate human physiology. This limits their accuracy in terms of human translatability.
Offering improved human relevance over-engineered cell lines, and therefore a better match for OOC assays, are iPSCs. iPSCs are artificially generated cells that are reprogrammed from adult cells. They can differentiate into any cell type in the body, and can be expanded in culture, however, iPSCs may not fully recapitulate the characteristics of mature primary human cells, and they do not offer the same degree of genomic stability as primary human cells. they are also prone to spontaneously differentiating into other cell types.
Primary human cells, however, are isolated directly from human tissue and have not been genetically modified or transformed in any way. Although this means that primary human cells cannot be expanded in culture, when used in OOC assays, they more accurately recapitulate human in vivo responses – especially where multiple tissue-resident cells are combined. Culturing primary cells under flow perfusion (to mimic the bloodstream) has been game-changing for primary cell culture. Flow perfusion extends longevity significantly (up to a month for primary human hepatocytes (PHHs)), compared to just a few days in static culture, which provides enough time to induce disease or perform in vitro chronic dosing studies. Comparability to in vivo responses can be demonstrated throughout by quantifying the activity of specific enzymes, (such as the P450 family for PHH), or the release of cell-specific proteins into the culture medium.
As the purpose of OOC (also known as microphysiological systems (MPS)) is to recapitulate human physiology as closely as possible, we purposefully choose to use primary human cells where available – although ultimately any cell type can be used in OOC models.
Handling primary human cells
Primary human cells have received their share of “bad press” relating to their handling, retention of their native identity and survival throughout an in vitro experiment. In the past, handling and culturing these cells was deemed a herculean task, since they are very particular in their requirements. Standard 2D static cultures using plastic consumables have hindered the performance of these challenging cells, however, more recent “ease of use” advances and the incorporation of flow perfusion by commercialized MPS/OOC platforms, have made their culture much easier and more accessible to scientists without prior experience.
Optimal primary cell handling techniques vary between cell types, but the most crucial steps to get right are the thawing and seeding procedures. For example, PHHs represent a “diva-like” cell type. They require specialised media during the thawing process as well as slow pipetting and wide bore tips when seeding. But don’t let this put you off, our standard operating procedures provide all the tips and tricks you need to ensure success.
The importance of pre-validating primary human cells for OOC
But, even if you have followed the thawing/seeding protocol to the letter, it doesn’t mean that your cells will perform well in 3D OOC culture. Trust us on this one! The importance of pre-validating cells before OOC use is often underestimated and overlooked. Use of non-validated cells in OOC assays risks poor microtissue formation, variable assay performance and unreliable data!
Over the last five years, we have tested over 50 PHH lots, from various cell suppliers, and >60% of them failed to meet our acceptable performance criteria, the majority failed to even form 3D liver microtissues. We calculate that identifying two donors that pass our validation procedures costs around $10-15K and 100+ hours of a scientist’s time – but this is time well spent. By using pre-validated cells, you will benefit from cross-experiment consistency and improved data robustness.
Why do we recommend purchasing pre-validated cells?
We have partnered with providers of high-quality cells, such as LifeNet Health LifeSciences, to offer a solution that provides you with access to convenient, off-the-shelf, high-quality OOC validated cells without the burden of in-house quality control experiments. Our portfolio of 3D validated cells enables you to successfully recreate our advanced in vitro liver-on-a-chip models in your laboratory, removing the risk of failed experiments due to untested cells. Yes, there is a mark-up on these OOC validated cells versus the usual price of purchasing primary cells directly, but this reflects all the hard work that goes on behind the scenes to ensure that we provide you with the right cells, and cell combinations, that enable you to succeed.
How to perform your own pre-validation studies
Although we encourage PhysioMimix OOC customers to use validated cells from our catalog where possible, we understand that certain geographic regions have import restrictions that negate this. Additionally, you may want to develop your own models from scratch, own an alternative solution or perhaps even a platform that has been developed internally. So, what do you need to consider when performing your own in-house pre-validation assays?
Firstly, it is crucial to remember that humans themselves show intra-species variability as demonstrated in a recent FDA poster using acetaminophen to induce liver injury. Population differences must be considered and can even be embraced to study differing drug responses in patient cohorts – such as the 25% of our global population that have fatty livers. Furthermore, depending on your study requirements, donor age, sex, ethnicity, as well as life exposures could be examined and carefully matched before donor selection. Aside from this inherent population-based variability, differing cell extraction methods between cell providers can introduce additional variability too.
To assess variability in donor-to-donor performance requires thorough testing using the OOC platform of your choice versus the future context of use (or application). Firstly, think about how many cell types you require in the model to achieve your assay objectives. For example, for drug metabolism studies we recommend keeping things simple by using a PHH-only model. When evaluating drug-induced liver injury, do you need to increase assay sensitivity by incorporating elements of the body’s innate immune system? If yes, include Kupffer cells (KC). When modeling liver disease, ask yourself which cell types are involved in inducing the disease’s pathophysiology and phenotype and factor these in – but remember that the length and cost of the validation process will also increase too. Next, you will need to identify which cell health and functional markers are important to monitor for the selected cell types, as well as pass/fail thresholds. We source this information from clinical data and past literature and adjust the thresholds to reflect the reduced cell numbers used in the model versus the human organ.
What does an example tri-culture cell validation process look like?
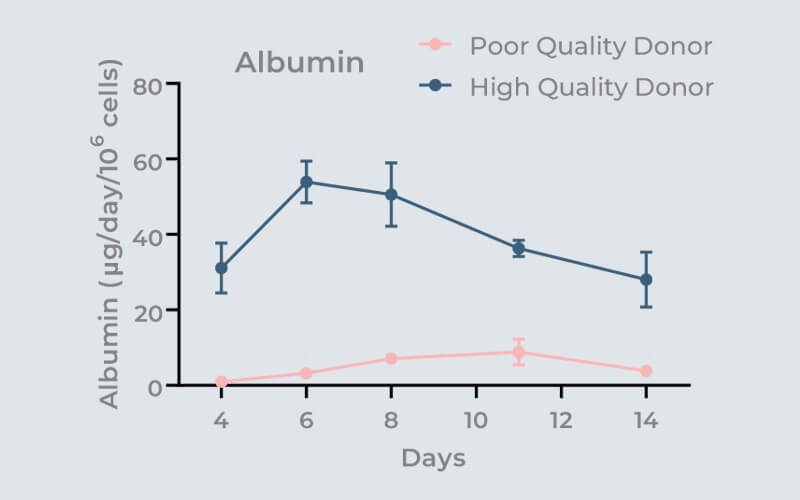
We have developed a model of the human metabolic disorder Non-alcoholic steatohepatitis (NASH), using PHHs, hepatic stellate cells (HSC) and KCs. Let’s use this as an example to walk you through a tri-culture validation process. Firstly, you need to pre-select a number (between 5-10) of PHH donors and identify the key cell health and functional markers that are required to compare each donor’s performance, including pass/fail thresholds. To assess tissue formation on scaffolds we also recommend that you perform tissue-specific immunofluorescent staining and subsequent confocal microscopy/analysis to visually confirm the quality of your 3D cultures. For PHHs, we subsequently quantify a variety of functional biomarkers, including LDH, Urea, Albumin and CYP3A4.
To ensure longevity of the liver tissue culture all selected PHH donors for a period of fourteen days using your OOC platform. Collect media samples every 2-3 days to monitor cell health and function to assess if the donor cultures remain within, or outside, acceptable limits at each time point and demonstrate sustained functionality. This process is normally run twice to ensure the reproducibility of the donor’s characteristics.
In a similar manner, the second step involves selecting several KC donors and testing their response to an inflammatory stimulus, in this case to Lipopolysaccharides (LPS). To assess their response, we quantify inflammation markers such as interleukine-6 (IL-6) and tumor necrosis factor-alpha (TNFα).
This is performed as a co-culture with one or two successful PHH donors for a period of 14 days whilst treating with LPS as a KC inducer. To ensure that the performance of co-cultured cells remains within acceptable limits, we assess PHH health and cell function, plus KC inflammatory responses, at different time points.
The third step is to source several HSC donors to co-culture with pre-validated PHHs and KCs. A triple culture experiment is set up to test multiple combinations of PHHs, KCs and HSCs, ideally creating distinct combinations of donors. During validation experiments we culture the cells in our high-fat media (HEP-Fat) to induce a NASH phenotype. As with previous stages we culture the experiment for 14 days to ensure longevity of the culture.
HSC inducers are then selected, such as transforming growth factor beta (TGFβ) which specifically activate HSCs. These are used to treat the cells to determine whether the co-cultures deliver a more advanced fibrotic phenotype once induced. Chosen endpoint assays with pre-determined pass/fail cut-offs need to include fibrosis markers e.g., Fibronectin, TIMP-1 as well as immunofluorescent staining for fat and HSC activation. Those triple-cultures that deliver stable performance (i.e., within the chosen criteria limits for each cell type) over the 14-day period are now validated for use.
While the validation process is logical and straightforward once you gain experience, the most crucial step in the procedure is the first. As previously mentioned, around 60-70% of PHH donors fail to culture in a 3D OOC environment. Additional cell types are much more likely to successfully seed when cultured with a good PHH donor. In our hands, approximately a quarter of triple culture combinations fail. To give you a heads up, you will need to budget tens of thousands of dollars and factor in a testing period of four to six months to run a study like this, however, for NASH, it is also possible to circumvent the process should you wish to!
NASH-in-a-box reagent kits
We have applied this validation principle to a unique product called NASH in-a-box, which is tailored for use with our PhysioMimix® OOC range of microphysiological systems. This kit not only offers 3D validated triple cultures comprising of PHHs, HSCs and KCs but also includes thawing media, quality control assay kits for liver microtissues, proprietary NASH-inducing cell culture media, Multi-chip Liver-12+ consumable plates and supplements for cell culture. Combined with software-guided protocols (which walk you through the experimental process step-by-step), the kit streamlines the entire workflow, enabling you to benefit from over a decade of our experience. Importantly, they also provide you with a choice – to spend your valuable time making new discoveries, or validating cells!
What’s next?
Our customers have benefitted hugely from using our portfolio of 3D validated liver cells, or NASH-in-a-box kit, to accelerate the pace of OOC adoption into their workflows.
As we diversify our range of models to include additional organs, we will continue to develop our portfolio of 3D validated cell and “in-a-box” kits, so if your application area is not currently served by our portfolio – watch this space for further developments, or contact us to register your interest.




