How to more accurately estimate human drug bioavailability?
Estimating the bioavailability of drugs in humans during preclinical development is necessary to set safe and effective doses in the clinic. Submitting bioavailability data is also a regulatory requirement.
Extrapolating PK parameters from animals to humans during drug development is challenging. The overall correlation between animal and human bioavailability is poor mainly due to differences in physiology and metabolic capacity.
Achieving high oral bioavailability is advantageous to reduce safety issues. Insufficient bioavailability in humans requires an increase in drug dose to be effective; however, this increases the risk of toxicity and side effects.
Our solution
We have developed a PhysioMimix® Bioavailability assay that uniquely recreates the combined effect of intestinal permeability and first-pass metabolism using PhysioMimix Gut/Liver-on-a-chip models. Our advanced in vitro human models recreate the function and fluidic interaction of the human gut and liver to emulate the dynamics of drug absorption through the intestinal barrier followed by their subsequent metabolism by the liver. The approach enables you to compare intravenous and oral compound dosing for the prediction of human oral bioavailability.
The assay delivers robust and reliable data to better inform the design of in vivo animal studies and validate in silico PBPK modeling. Its insights facilitate the improved estimation of preclinical bioavailability for more informed decision-making ahead of clinical trials.
Recreate the assay in your own laboratory using PhysioMimix Core or access via our ADME Contract Research Service.
Take a tour with our bioavailability animation

Read publication
A primary human Gut/Liver microphysiological system to estimate human oral bioavailability.
Studying drug bioavailability
Limitations with current techniques
- Animal models fail to accurately predict human bioavailability
- Animal models have varying expression levels for enzymes that drive drug metabolism in humans
- Simple in vitro models cannot model the systemic effects of the drug
- In silico models are dependent on quality input parameters that rely heavily on early in vitro and animal studies
Advancements with PhysioMimix Core
- Enables the direct, accurate measurement of bioavailability using a human-relevant system
- Perfusion of microtissues promotes the metabolic capacity of cells
- Allows the combined effects of intestinal permeability and liver metabolism to be explored
- Enables estimation of gut and liver ADME parameters by combing with computational modelling tools
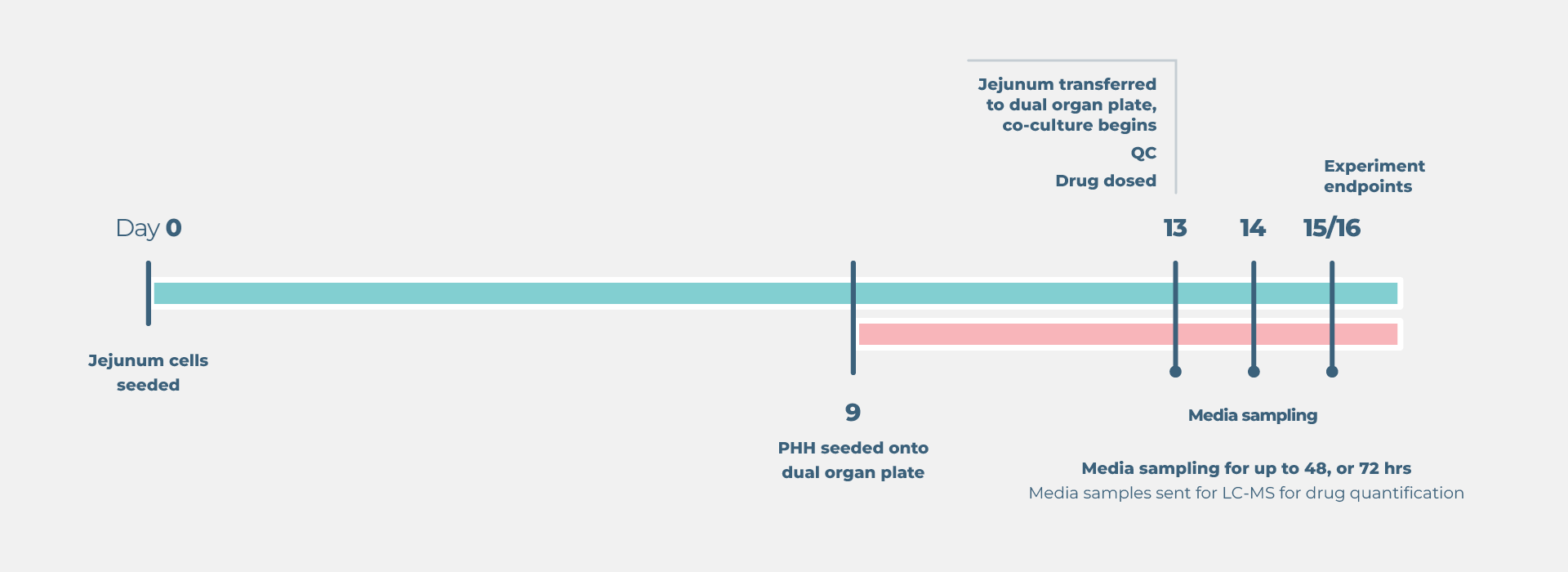
End point measurements
Longitudinal and endpoint measurements include (but not limited to):
Liver: Functionality biomarkers
- Cytochrome P450 enzyme activity
- Lactose dehydrogenase (LDH) release
- Albumin production
Gut: Functionality biomarkers
- Trans epithelial electrical resistance (TEER)
- Lactose dehydrogenase (LDH) release
Profiling analysis
- Media samples sent to LC/MS for bioanalysis
- Parent drug concentration over time
- Metabolite concentration over time
- Area under the curve estimation
Optional profiling analysis
- Microscopy biomarker analysis
- Transcriptomics
ADME parameter predictions
Combine with computational modelling to predict the following from a single experiment:
- Liver clearance (CLint, liver)
- Gut clearance (CLint, gut)
- Gut permeability (Papp)
- Efflux ratio (E)
- Fraction absorbed (Fa)
- Fraction escaping gut metabolism (Fg)
- Fraction escaping hepatic metabolism (Fh)
- Oral bioavailability (F)
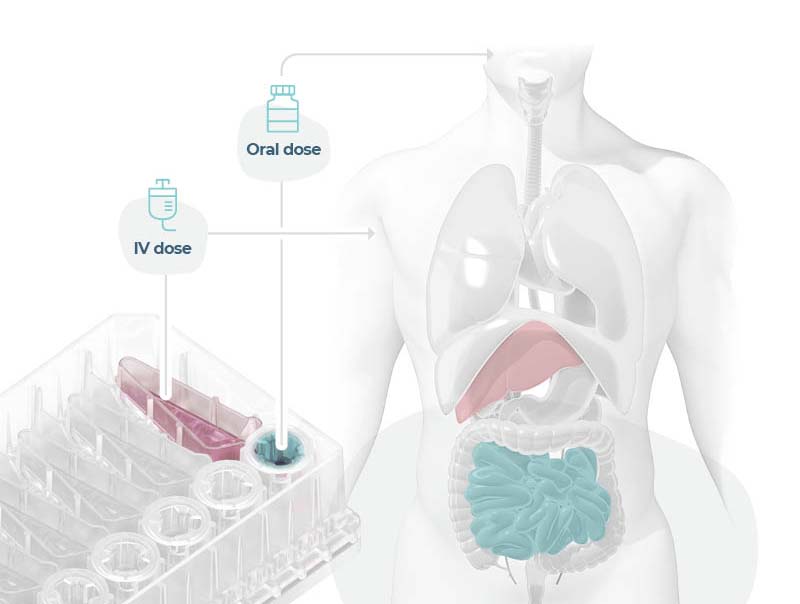
Modeling an orally administered drug’s route of entry
Uniquely recreate the combined effect of intestinal permeability and first-pass metabolism in vitro using human Gut/Liver-on-a-chip models to more accurately estimate oral bioavailability.
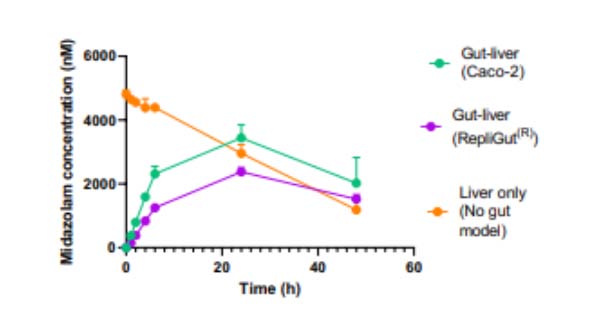
In this example, we demonstrate Midazolam metabolism by the liver in isolation versus the combined contribution of both organs using two Gut/Liver-on-a-chip models (Caco-2, or primary human RepliGut®).
Midazolam concentration in the dual-organ model was determined over time following either apical dosing of gut tissue (oral) or in liver-only wells (IV) and sampling media from the liver compartment. Improved estimations were observed using the primary RepliGut/Liver model.
Learn more in our application note
Connecting the gut and liver: A human relevant dual-organ microphysiological system connecting the gut and liver for preclinical profiling of oral bioavailability. Read appnote
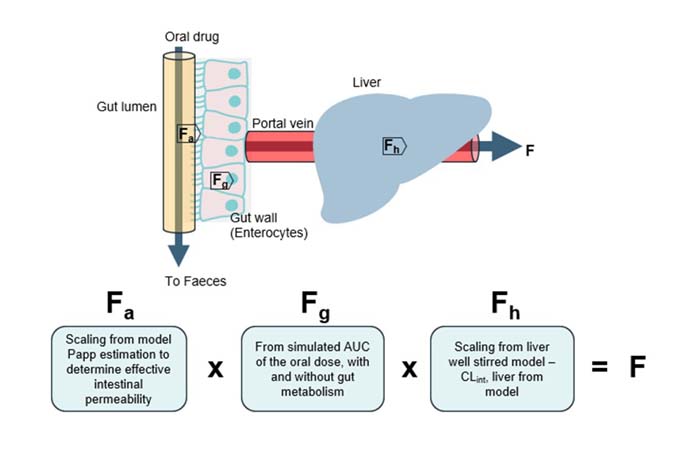
Combine with computational modeling to predict human bioavailability
Our innovative in vitro approach combines our Gut/Liver-on-a-chip (also known as a Gut/Liver microphysiological system) with a mechanistic mathematical model to accurately predict key ADME parameters and oral bioavailability. By estimating:
- Fraction absorbed (Fa)
- Fraction escaping gut metabolism (Fg)
- Fraction escaping hepatic metabolism (Fh)
We can predict human oral bioavailability (F) – offering a powerful tool for drug development and optimization. Read more in our publication Abbas et al., 2025
How does our PhysioMimix Bioavailability assay compare?
A wide range of preclinical research and development tools is available for scientists.
How does the Bioavailability assay compare to traditional approaches?
| Liver microsome / PHH suspension | Gut-Caco-2 | Animal | PhysioMimix Bioavailability | |
| Human | ||||
| Human-relevant CYP activity | ||||
| Intestinal absorption & hepatic clearance in one | ||||
| Throughput | ||||
| High content | ||||
| Cost | $ | $ | $$$ | $$ |
Explore our Gut/Liver-on-a-chip models
Interconnect and maintain the high functionality of Gut-and Liver-on-a-chip models to recapitulate human gut barrier integrity and metabolic capacity in vitro.
Access our ADME service
Get instant access to PhysioMimix Bioavailability Assay via our CRO Service. Through a collaborative approach, our experts work with you to plan and execute your study.
Standard and bespoke projects are carried out by our dedicated team of scientists in our CRO facility, providing you with actionable data within weeks.
Add PhysioMimix Core to your lab
Harness the power of PhysioMimix Core in your own lab.
With a growing community of users and support from our experts, there has never been a better time to transition into 3D cell culture.
Frequently asked questions
Why are animals poor predictors of bioavailability?
There are many reasons, however, the repertoire and expression of enzymes that drive human drug metabolism differ compared to those of commonly used animal species. This is also true for transporters that are responsible for the uptake or efflux of drugs through the small intestine. In addition, there are differences in the physiology of certain animal species compared to the human. For example, rats do not have a gall bladder – an important component of the hepatobiliary system.
In their 2014 publication, Musther et al, demonstrate how poorly animals predict this key parameter. For 184 drugs investigated, the R2 value of animal model estimations versus actual clinically derived human bioavailability were shown to be 0.34.
What are the benefits of your approach to human bioavailability testing in vitro and could it replace the need for animals?
We expect animal models to continue to be an important part of ADME studies for the foreseeable future, however, the complementary use of the PhysioMimix Bioavailability assay will add confidence to the data generated with animals or query their findings.
In their 2014 publication, Musther et al, demonstrate how poorly animals predict this key parameter For 184 drugs investigated, the R2 value of animal model estimations versus actual clinically derived human bioavailability was shown to be just 0.34.
A deeper and earlier knowledge of human bioavailability enables issues to be addressed before the start of costly preclinical studies. In vivo models are only an approximation of what’s likely to happen in the human as they lack human relevance; and traditional in vitro assays are limited by physiological relevance. Advanced human models, however, address these limitations, enabling you to bridge the gap to confidently select safer and more effective drug candidates.
How many parameters must be included when using physiologically-based pharmacokinetic (PBPK) modeling to extrapolate data generated by the human bioavailability in vitro assay? Do you have to increment each different flow?
For predictive modeling, two main parameters are required: hepatic clearance rate and compound permeability through the intestinal barrier. We are working on a mathematical model that will take data generated from the Multi-chip Dual-organ consumable plate and generate fittings for the parameters. Flow rates can easily be adjusted by the user via the PhysioMimix Multi-organ System’s controller unit, which allows us to understand the effects of different mixing rates.
To learn more, read our Multi-Organ Application Note: Drug metabolism in a gut-liver microphysiological system. Note this study utilizes our Caco-2 Gut/ Liver-on-a-chip model
Have you characterized the liver CYPs in the model used for human bioavailability testing in vitro, and is their expression the same as in humans?
We have characterized the activity of the CYP3A4 enzyme, one of the main drivers of drug metabolism in humans, for quality control purposes during culture to ensure that there is sufficient and maintained activity throughout the experiment. This has also been independently validated by the CDER group at the FDA in the following study by Rubiano et al, 2021.
How can mathematical modelling be utilized when performing human bioavailability testing in vitro?
We can take data generated from the PhysioMimix® Bioavailability assay kit: Human 18, and conduct parameter fitting to estimate the hepatic clearance rate and permeability of drugs across the intestinal barrier. Here, parameter fitting is the process of computing a model’s parameter values from measured values. By utilizing mathematical modelling, the data generated from one bioavailability experiment can be maximized.
We recommend reading our recent publication (Abbas et al., 2025) where we demonstrate how to combine data derived from the primary Gut/Liver-on-a-chip model in our kit (also known as a Gut/Liver microphysiological system) with a mechanistic mathematical model to generate organ-specific pharmacokinetic parameters and estimate human oral bioavailability and its components: the fraction absorbed (𝐹𝑎), the fraction escaping gut wall elimination (𝐹𝑔), and the fraction escaping hepatic elimination (𝐹ℎ). Bioavailability prediction using mechanistic mathematical modeling of experimental data is available via our ADME Contract Research Service.
Additionally, this publication by Roche (Milani et al., 2022) describes the mechanistic modeling of mycophenolate mofetil experimental data from a Caco-2 Gut/Liver-on-a-chip.
Can the Gut/Liver human bioavailability in vitro assay be used to profile the bioavailability of low clearance compounds?
Yes, using the Gut/Liver-on-a-chip, or microphysiological system (MPS) as it is also known, we have demonstrated that bioavailability can be profiled for low clearance compounds, which are defined as drugs that have an intrinsic clearance rate of <5ml/min/kg.
To learn more, read our Multi-Organ Application Note: Drug metabolism in a gut-liver microphysiological system. Note this study utilizes our Caco-2 Gut/ Liver-on-a-chip model
Which animals have you compared your in vitro human bioavailability test to in terms of allometric scaling?
We have not applied allometric scaling to data generated using either the Liver-on-a-chip or the Gut/Liver-on-a-chip.
What is the clearance mechanism of the compounds that you have explored. Were any transported substrates included? And would you be willing to share the names of the compounds you tested?
Using the primary Human RepliGut/Liver-on-a-chip model, we have tested Temocapril and Midazolam, who’s human ADME properties were not adequately predicted by existing in vitro, or in vivo models. Temocapril is a pro-drug designed to be resistant to intestinal hydrolysis. It is metabolized to Temocaprilat by carboxylesterase 1 (CES1). The isoenzyme pattern in human is well-characterized with the liver and intestine expressing CES1 and CES2 respectively.
In Caco-2 cells, there is a miss-match as CES1 is predominantly expressed, resulting in an overestimation of drug clearance. In contrast, the more human representative ratios of both CES1 & CES2 expression by the primary human RepliGut model make it more relevant for pro-drug studies. Midazolam predominately undergoes intestinal clearance by the CYP3A4 enzyme. When compared to the equivalent Caco-2/Liver-on-a-chip model, the primary RepliGut/Liver-on-a-chip model delivered oral bioavailability estimations that more closely represented human clinical observations
For further reading please view our latest Application Note
When using primary cells for human bioavailability testing in vitro, how many donors do you use to account for genetic variability within a study?
It is well known that the repertoire and expression of enzymes that drive metabolism differ with genetic diversity. One of the benefits of MPS over in vivo animal studies is that you can run experiments using primary cells from multiple donors to account for/investigate the impact of genetic diversity.
Does your in vitro human bioavailability assay allow for a calculation of the fraction absorbed (Fa), fraction escaping the gut (Fg) and fraction escaping hepatic metabolism (Fh)?
Yes, by combing the bioavailability assay with computational modelling we can estimate Fa, Fg and Fh. Please read the following publication for more information (Abass et al., 2025). Bioavailability prediction using mechanistic mathematical modeling of experimental data derived from our PhysioMimix Bioavailability assay is available via our ADME Contract Research Service.
Is your approach to human bioavailability testing in vitro compatible with combination dosing?
Combination dosing Gut/Liver-on-a-chip models (also known as Gut/Liver microphysiological systems) has not been tried, but where LC-MS analysis is able to differentiate between compounds, the bioavailability of drug combinations could be estimated using a modified version of this approach. The benefit of MPS is that many more dosing regimens and concentrations can be tested to finely tune estimations compared to animal studies.
Contact us for more information.
When performing human bioavailability testing in vitro using your approach, is there any serum in the culture medium?
Serum-free media is used during the human bioavailability in vitro experiment to limit drug binding with proteins. Additionally, it is worth noting that we avoid the use of Polydimethylsiloxane (PDMS) in our Multi-Chip Dual-organ consumable plates, which are made using Cyclic Olefin Copolymer (COC) instead.
How do you minimize non-specific binding when performing human bioavailability testing in vitro?
By using serum-free media and PDMS-free consumable plates. Although PDMS is widely used, it has limitations. Unfortunately, PDMS has very high non-specific binding properties, which poses a challenge for human bioavailability in vitro testing. Customers report that up to 70% of drugs can bind to the plastic, which impacts data accuracy. It is possible to saturate PDMS plasticware ahead of experiments, however, this isn’t ideal as it adds more complexity with respect to assay set-up and poses a contamination risk. When developing our Multi-chip consumable plates, we decided to move away from PDMS and use Cyclic Olefin Copolymer (COC) instead. For cell culture applications, COC, is currently one of the most inert materials in the market. and is therefore more suitable for estimating bioavailability in vitro, although we advise performing exploratory studies to check for non-specific binding should there be concerns.
We also recommend running a cell-free condition, where the compound is added but no cells are seeded in the Gut/Liver-on-a-chip. This allows users to monitor and consider any drug loss. Whilst we expect drug binding to the COC plastic to be low, we understand that it is important to show this experimentally, enabling increased confidence when testing human bioavailability in vitro.
Please read the following references to learn more: van Midwoud et al, 2012, Tsamandouras et al., 2017, McAleer et al, 2019.
Is your Lung/Liver model suitable for human bioavailability testing in vitro?
Our Lung/Liver model is very exciting, and it’s something that we can apply to (more or less!) anything people are interested in. In the first instance, we designed this dual organ system for COVID-19 research, as funded by our Innovate UK grant. In particular, we looked at the crosstalk between the lung and liver when we infected the lung with COVID-19 pseudoparticles. Through this, we’ve been able to elucidate over time how the liver responds to inflammatory signals given by the lung during the challenge. In the future, this could also be applied to drug studies, whether that be ADME, toxicity, bioavailability or pharmacology – there’s a whole host of different applications that could be applied to the multi-organ system.
If you do not find the answer to your question listed, please contact us