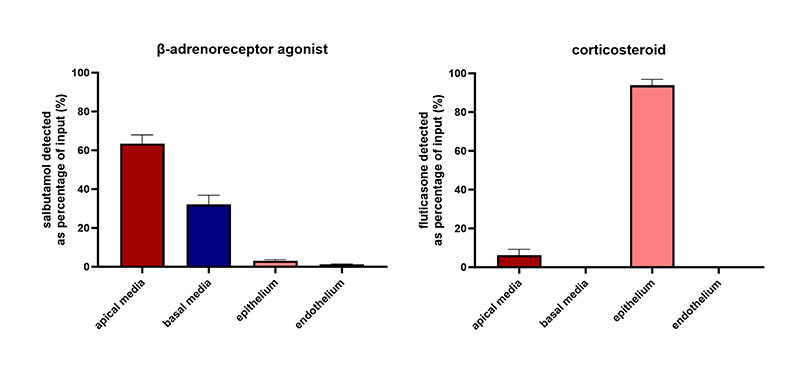
Lung on a chip models
Breathe life into your data with Lung on a chip models
Our predictive human Lung-on-a-chip models (microphysiological systems) are cultured in Multi-chip Barrier plates by PhysioMimix® Core Systems.
Recreate the multi-cellular architecture of the airways and alveoli, enabling fully predictive disease modeling and drug absorption studies.
Getting started is easy. We provide fully validated SOPs for lung-related applications or access via our ADME Contract Research Services.
Human alveolar microphysiological system
Human bronchial microphysiological system
What are the advantages of Lung on a chip models?
Fluidic flow
Enhances differentiation by providing nutrients, oxygen, and biomechanical stimuli
Pseudostratified epithelium
Formation of a human-relevant barrier to insults and drugs

Functional tissue
Bronchial model goblet cells produce mucus and alveolar model AT2 cells produce surfactant
Co-cultures increase complexity
Combine additional pulmonary or immune cells to further recapitulate the lung niche
Physiological dosing
Open well format allows liquid or aerosolized dosing using commercially available devices

Increased depth of analysis
Large sampling volumes and tissues allow enhanced analysis and translation to clinical results
Flexible cell compatibility
Primary or stem-cell-derived cells, cell lines or tissue slices can be utilized
Robust and reproducible
Delivers reliable data via low inter- and intra-assay variability
Human in vitro bronchial microphysiological system
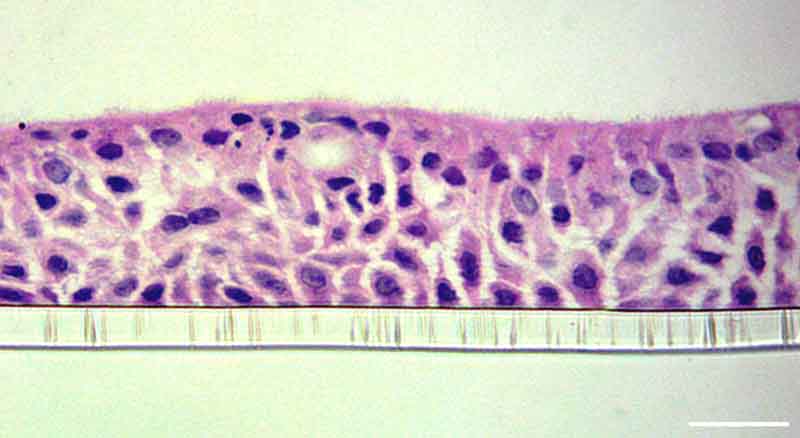
Primary human bronchial epithelial and endothelial cells are cultured on either side of an insert scaffold at air-liquid-interphase (ALI) to produce a pseudostratified epithelium over 14 days.
Culturing in perfusion enhances the differentiation of the bronchial epithelium with a functional mucociliary barrier retained for long-term culture.
Human in vitro alveolar microphysiological system
Primary human small airway epithelial and endothelial cells are cultured either side of an insert scaffold at air-liquid-interphase (ALI) over 14 days to form tissue with alveolar-sac architectures.
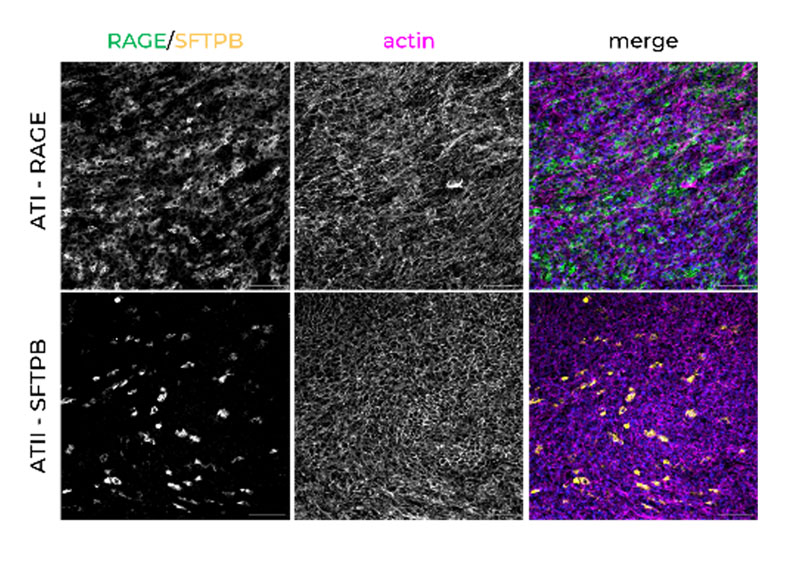
Alveolar Type I and II cells are maintained at appropriate human populations over time in the perfused conditions.
Fully validated Lung on a chip models
| Lung models | Cell type | Applications | Required products |
|---|---|---|---|
| Alveolar model | Epithelial and endothelial cells | Drug absorption Pulmonary Infection | PhysioMimix Core System, Barrier plates |
| Bronchial model | Epithelial and endothelial cells | Drug absorption Pulmonary Infection | PhysioMimix Core System, Barrier plates |
Lung on a chip Applications
Frequently asked questions
How long can Lung-on-a-chip tissue be cultured by PhysioMimix® Core systems?
In general, our lung MPS are cultured for 14 days for optimal differentiation. We have cultured tissues up to 28 days, and the barrier integrity, phenotype, and function remained stable. Due to the open-well format of the PhysioMimix Multi-chip Barrier-12 plates, repeat dosing and sampling over extended periods is straightforward.
What cell types are represented in your Lung-on-a-chip models?
We have two Lung-on-a-chip – also known as Lung microphysiological systems (MPS) – an alveolar and bronchial model. The human alveolar model contains primary cells which differentiate to type I and type II pneumocytes over 14 days, with the formation of alveolar sac-like formations and the production of surfactant by stable type II cells. The bronchial model forms a functional pseudostratified epithelium containing ciliated, goblet, club, and basal cells. Both models can also be co-cultured with pulmonary endothelial cells to reproduce the pulmonary-vasculature interface.
For a visual representation of the models, please view the two animations that bring our alveolar and bronchial models to life (above).
Is it critical for 3D Lung-on-a-chip models to have an air-liquid interface (ALI)?
Yes, this is particularly important during differentiation of the models, as this recapitulates the environment that the epithelial cells experience in the human body. This, teamed with the added oxygen and nutrition provided by the fluidic flow from PhysioMimix Core Systems, allows the lung microphysiological systems to form the most human-like epithelium possible. For challenges with stimuli, pathogens or drugs, our lung MPS models can be configured for use with and without air-liquid interfaces.
Why is perfusion important in establishing and testing Lung-on-a-chip models?
Perfusion serves two main functions. Firstly, mechanical cues. We live because of mechanical forces and mechanical stresses – our heartbeat, our movements, our muscle tension – provide critical and fundamental biomechanical cues to ourselves, which trigger many regenerative processes, especially in our lungs. By introducing the shear stresses of the microcirculation into our models, it adds value by mimicking human physiology. Secondly, the movement of media allows additional oxygenation and nutrition to be transferred to the epithelium in ALI conditions. Perfusion also moves the molecules which are secreted by cells – cytokines, proteins, vesicles – as well as any challenge articles – drugs, pathogens – around the culture to avoid negative gradients that would otherwise be seen in static cultures.
Professor Chrzanowski’s (Faculty of Medicine and Health, University of Sydney Team explored the positive effects of perfused versus static lung-on-a-chip models in detail within this publication Phan et al.2023. CN Bio has also explored the importance of perfusion in this Application note
Can any commercially available aerosoliser or nebuliser systems be used with your Lung-on-a-chip?
By using aerosolized/nebulizers, the dissolution properties of drugs can be determined. To date, we have focused on the absorption and permeability of drugs using liquid dosing. However, as our lung microphysiological system models are built on 24-well sized inserts, such as Transwells, they are well adapted for use with many commercially available aerosolization/nebulization systems for this application. The inserts can be temporarily removed from the Multi-chip Barrier plate, placed into these systems for drug or stimuli challenge and then returned to the PhysioMimix system.
Are alveolar cells embedded in a 3D matrix or do they assemble air sacs spontaneously at Air-Liquid Interphase (ALI)?
We do not use 3D matrix/scaffolds in these models. Cells are plated directly onto collagen-coated Transwell® membranes and left to differentiate at ALI. The air sac-like structure formation is not solely due to ALI culture, although this certainly aids cell differentiation. We do not see any air sac-like formation in tissues that are cultured in static conditions, so we attribute the formation of these structures to the benefits of fluidic media flow controlled by the PhysioMimix Core System.
What applications can your Lung-on-a-chip models be used for?
The U.S. Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER) used our models to evaluate the safety and ADME properties of inhaled medications. At CN Bio, we also offer the ability to study drug permeability across lung barriers and bioavailability via our ADME Contract Research Service.
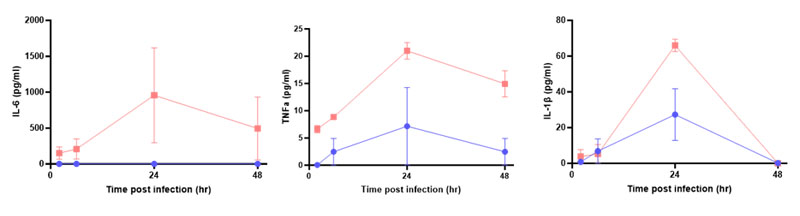
PhysioMimix Core has been successfully used by the Chrzanowski Group at the University of Sydney to model the respiratory disease chronic obstructive pulmonary disease (COPD) to test the efficacy of novel therapeutics. Obviously, the other area to explore is infection models, including pulmonary infection. Internally, we would like to complete further experiments on BSL-2-safe respiratory pathogens, such as the influenza virus, and use our multi-organ plate to understand crosstalk between the lung and liver during these infections. We would also like to include more immune cell types in these models to increase physiological relevance and, therefore, the models’ ability to predict responses.
Here are recommended resources to find out more:
Webinar: Every breath you take (ADME)
Webinar: A breath of fresh air (COVID 19 & COPD)
Publication: COPD Phan et al., 2023 Advanced pathophysiology mimicking lung models for accelerated drug discovery
Application Note: Alveolar and Bronchial Microphysiological Systems for respiratory infection research and therapeutics evaluation
Presentation: Efficacy focus with Claire Caygill
Can Lung-on-a-chip models replace or reduce animals in research?
The key element here is that some animal models do not adequately represent human physiology or human disease. Technologies such as humanized mice have closed the gap, but limitations remain. In vitro lung-on-a-chip models mimic human physiology, allowing us to test things in a more physiologically relevant way. The formation of the human-specific-, or human mimicking, structures within these models remain isolated from a whole system, i.e. there’s no enzymatic regulation and there’s often only one humanized organ, or limited interaction with other organs in multi-organ systems such as the Lung/Liver. However, these in vitro models enable us to test multiple formulations for safety and efficacy in a much faster and much more representative way than some animal models. Obviously, they will not eliminate the use of animals, especially in the short term, but they can reduce the number of animals we need to test our formulations in. The reason for this is that, after testing in these human predictive models, we will have a higher degree of confidence. It is feasible that these models will become a prerequisite before human clinical trials, as they provide key answers to drug safety and efficacy questions and for optimizing treatment regimens, whether single dose or repeated doses are required.
Can different types of inserts be used in the Barrier-12 plate?
The lung microphysiological systems we have developed are based upon 24-well-sized Transwell inserts with 0.4 μm pore size. However, if you wish to develop an MPS model with varying pore sizes, materials or suppliers, the Multi-chip Barrier plates are flexible to other designs. As an example, if larger pore sizes are required for e.g., immune infiltration applications, the epithelial/endothelial cultures may require optimisation prior to use. Other suppliers may be used, but the size and depth of the inserts must be considered prior to use in the perfused system, as this could affect the flow paths and resulting shear stress.
Why did you use the THP 1 cell line instead of primary monocytes in your Lung-on-a-chip model?
We began immune coculture model development using the human leukaemia monocytic cell line (THP-1) because they are easy to culture compared to primary monocytes; however, we plan to use primary immune cells in the future. Ultimately, we hope to recirculate peripheral blood mononuclear cells (PBMCs) in the basolateral side of the model to recapitulate the peripheral immune population, particularly for use in infection studies.
A customer of ours, Professor Wojciech Chrzanowski, Faculty of Medicine and Health, University of Sydney, has developed his own pulmonary models on the PhysioMimix system, first using immortalized cell lines and then primary cells. Professor Chrzanowski used immortalized cell lines to optimize conditions, and to get started with the PhysioMimix Core System and then switched to primary cell models for safety and efficacy assessments. Primary cell use enabled their team to recreate models in the laboratory that more accurately represent human physiology. They differentiate properly, they form cilia, they secrete mucous, and they form functional lung mimicking structures. The additional advantage of using primary cells is that they can be taken directly from patients to perform personalized screening, or personalized testing of formulation preparations for specific patients.
Here are some recommended resources to find out more:
Webinar: A breath of fresh air
Publication: COPD Phan et al., 2023 Advanced pathophysiology mimicking lung models for accelerated drug discovery
Have you characterized single cell transcriptomics of the Lung-on-a-chip model?
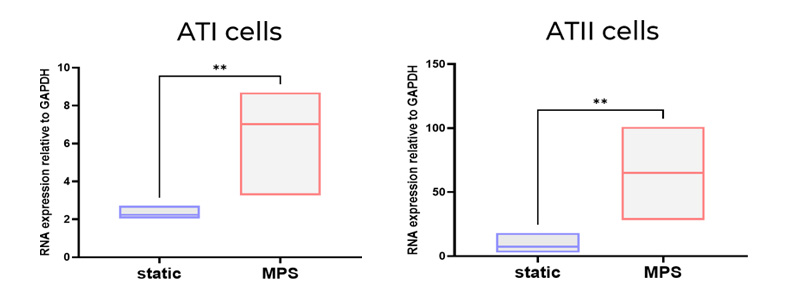
No, we have not undertaken single-cell transcriptomics in the lung models – but it’s something we would like to do in the future to get a full picture of the model and how it compares to the human lung. We have performed qPCR analysis to demonstrate the presence of cell markers of key cell types (AT I / II markers in the alveolar model. Goblet, Club cell and ciliated epithelium markers in the bronchial model). This analysis showed an upregulation of these cell markers in the tissues cultured by the PhysioMimix Core System. Large differences in gene expression have also been quantified using microscopy. When we add further complexity to the model (e.g., via the incorporation of endothelial cells), we observe further upregulation of these cell markers, which is indicative of enhanced cell-cell communication and a more physiologically relevant phenotype.
Find out more in our recent webinars A breath of fresh air and Every breath you take, and Application Note: Alveolar and Bronchial Microphysiological Systems for respiratory infection research and therapeutics evaluation
Can you examine O2 utilization in the flow layer of Lung-on-a-chip model
Yes, it would be possible to embed an oxygen probe/sensor within the microphysiological system to monitor the oxygen level. As the PhysioMimix Core System’s Multi-chip Barrier plate has an open well, accessible format, this would be relatively simple to do. It is something we would like to include in future iterations of the model/Multi-chip Barrier plates, where we could also include other internal probes, for example, to measure barrier integrity (TEER). Doing so would allow us to learn more about the models in a highly quantitative, live and unobtrusive manner.
In-house verification of the primary cell lots is required for your Lung-on-a-chip models. What does a good cell lot look like and what qualifies it as good for microphysiological system work?
Ensuring we have a good supply of quality commercially available primary cells for use with PhysioMimix Core Systems is something that we focus a lot of time and effort on. When primary cells arrive, the first thing we do is to carefully look at the structures that they form in our lung-on-a-chip/lung microphysiological systems (MPS). Firstly, we ensure they can form a good barrier by looking at transepithelial electrical resistance (TEER) over several weeks, as well as probing with molecules such as lucifer yellow for apparent permeability measurements.
We also focus on the cell phenotypes formed after differentiation at the air-liquid interface (ALI), particularly the alveolar and bronchial epithelial cell phenotypes, to ensure they are forming lung tissues that resemble the human lung. For example, when thinking about the alveolar model, we ensure that the cell population remains at a good ratio of alveolar epithelial type I (ATI) to alveolar epithelial type 2 (ATII) cells. In other model systems, this balance is often skewed towards an ATI alone population, which is not representative of in vivo alveoli.
How are the cells used to generate lung-on-a-chip models representative of a broad group of humans, or the general population?
This is an interesting and important point. It’s something the OOC/MPS community is focused on. Being able to perform individual “patient-on-a-chip” assays and broaden out the scope of assays to better represent the variety of responses we would see within a population in the clinic is possible using an OOC approach.
So far, we have used a range of healthy donors from commercial cell suppliers. We work very closely with our cell providers to ensure access to a large range of donors with different properties. This is something we will continue to focus on and strive for further diversity as our suppliers’ technology and donor banks increase. It is important to get a range of genders, ages and ethnicities to ensure that when you’re making predictions using lung MPS, you are representing a whole population, not just a single person. This is a vital step for technology providers to get right in support of OOC/MPS use for regulatory purposes.
If you do not find the answer to your question listed, please contact us