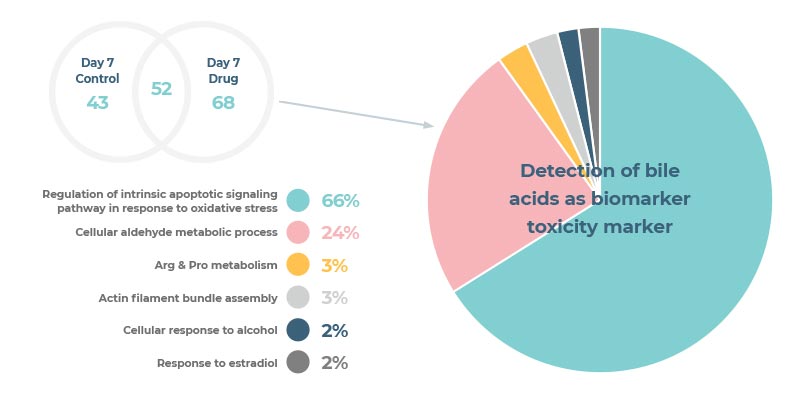
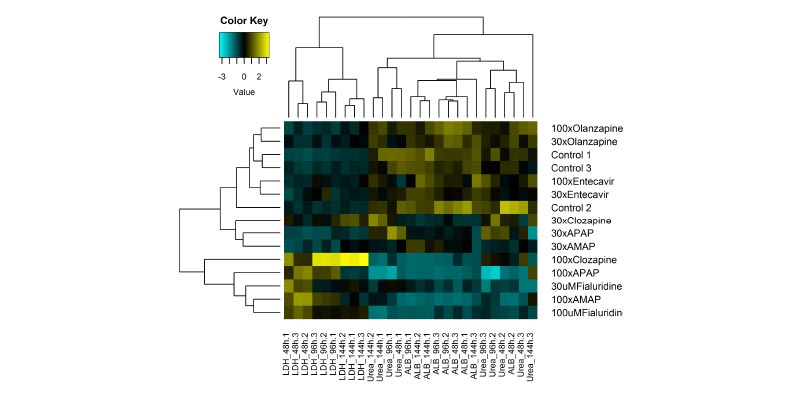
A sensitive in vitro hepatotoxicity assay that accurately predicts human DILI
DILI remains a major cause of drug attrition, due to the limitations of in vitro hepatotoxicity assays and in vivo models
Most medications are metabolized in the liver, which makes it one of the primary tissues affected by adverse drug reactions. Earlier identification means promising drugs can undergo structural modifications to address toxicity issues and lower attrition rates. To do so, preclinical in vitro hepatotoxicity assays with greater human relevance and sensitivity are required.
Our solution
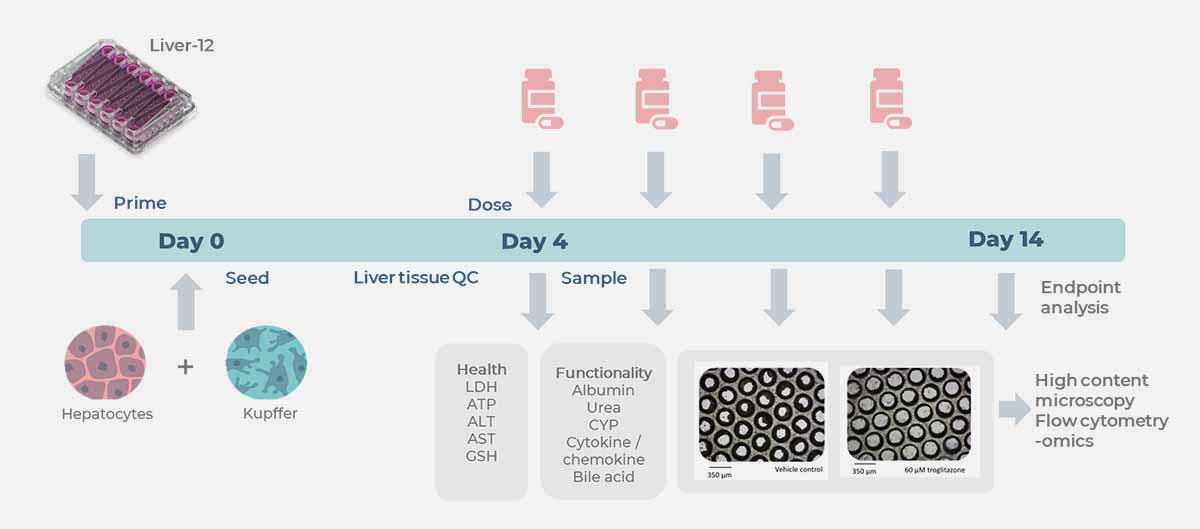
Utilizing our Liver-on-a-chip model, the PhysioMimix DILI Assay allows you to predict human liver responses to acute and chronic drug exposure in the presence or absence of underlying disease.
This in vitro hepatotoxicity assay consists of human primary liver parenchymal and non-parenchymal cells (NPCs) cultured in 3D under perfusion to form tissues from which it is possible to detect functional liver-specific endpoints (including clinical biomarkers such as albumin and ALT/AST). The assay enables the sensitive assessment of compound toxicity with full dose–response curves and, by incorporating Kupffer cells, allows for the evaluation of immune-mediated toxicity.
Both acute and chronic exposure to compounds can be assessed by comparing responses to single- and multi-dosed tissues for up to four weeks. The assay produces highly functional and metabolically active liver tissues for DILI screens to give deep mechanistic insights into the toxicity profile of a compound.
The DILI assay supports:
- More informed stop-go decisions before preclinical studies via superior in vitro to in vivo translatability (IVIVT) and clinical endpoint reporting.
- Investigative toxicology evaluations during preclinical or clinical studies to understand the mechanism of detected hepatotoxicity and inform risk mitigation strategies.
The benefits
Refine pre-clinical experimental design
More effective, physiologically relevant models deliver human translatable mechanistic insights that enable you to narrow down compound selection before progressing to in vivo testing to reduce animal use and save costs.
Be better prepared for the clinic
Mimicking human physiology in ways that can’t be achieved traditionally in vitro provides unrestricted access to testing advanced drug modalities where no viable in vivo models exist.
Studying DILI
Limitations with current techniques
- Poor in vitro to in vivo translation
- Fail to provide deep mechanistic insights into the cause of toxicity
- Many advanced in vitro models are low throughput limiting the number of data points
- Challenging to run chronic exposure assays
- Production of toxic phase I and II metabolites is not possible
Advancements with PhysioMimix OOC®
- Translate data from the lab to the clinic by using clinical biomarker reporting
- Assess multiple cellular endpoints to determine the mechanism of toxicity
- Generate full dose–response curves for multiple compounds per plate
- Long-term liver cultures facilitate prolonged repeat dosing for weeks
- Correlate the production of toxic phase I and II metabolites with cell health measurements
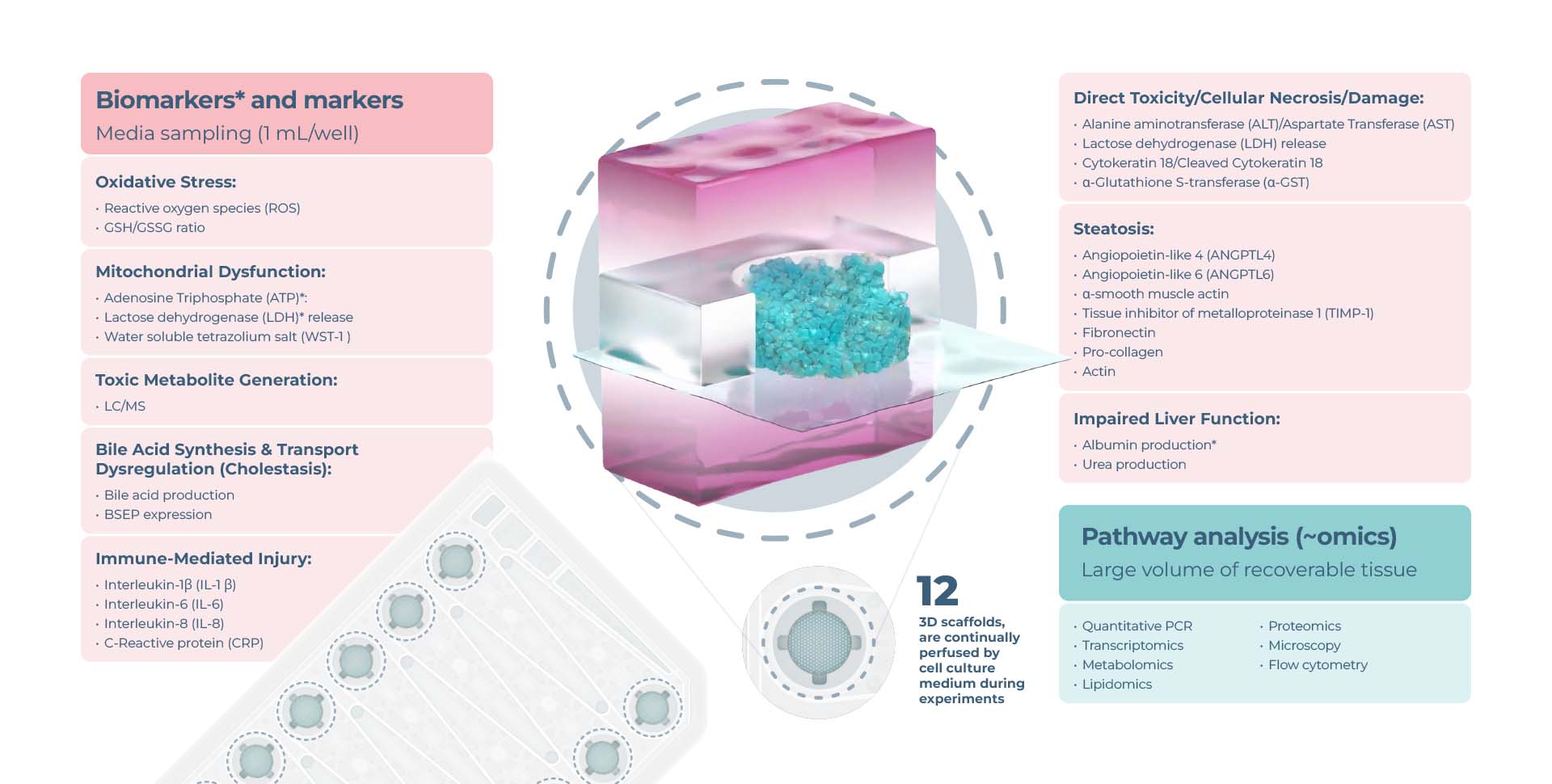
Limitless endpoint measurements
View the drop-downs for standard DILI assay longitudinal and endpoint measurements from recovered media and liver tissues.
Expanded options for pathway profiling are in the graphic below:
Functionality biomarkers
- Cytochrome P450 enzyme activity
- Albumin production
- Urea production
Clinical liver health biomarkers
- Lactose dehydrogenase (LDH) release
- Adenosine Triphosphate (ATP)
- Aspartate Transferase (AST)/Alanine amino transferase (ALT)
Optional profiling analysis
- Quantitative PCR
- Transcriptomics
- Oxidative stress
- Mitochondrial dysfunction
- Steatosis
- Bile acid synthesis/ transport dysregulation
- Inflammatory response
Proven predictability, reliability and robustness
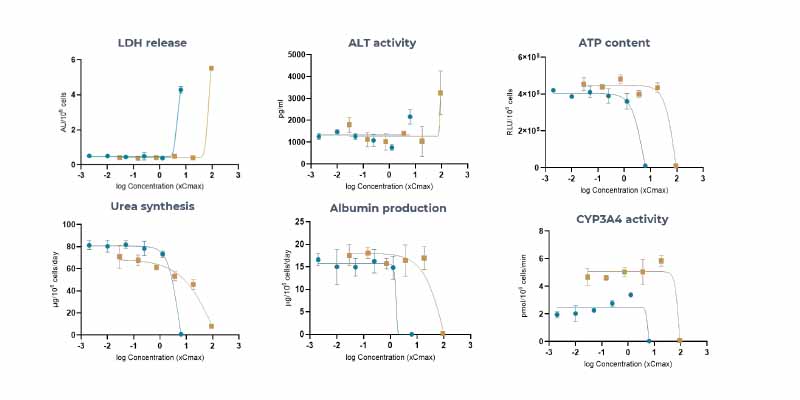
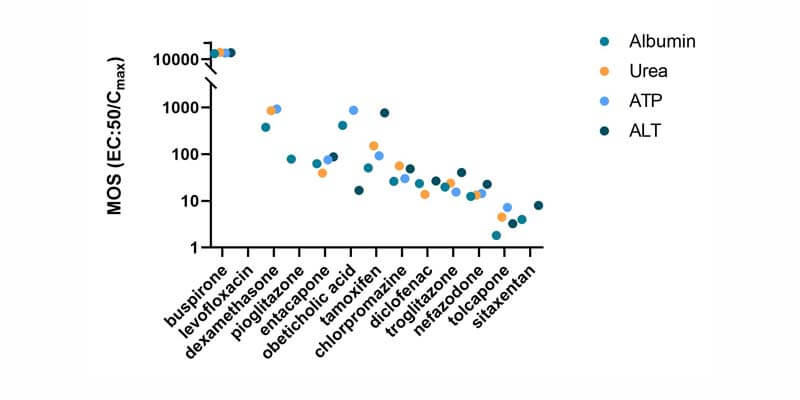
Using a panel of IQ MPS Consortium recommended reference compounds, the PhysioMimix DILI assay delivered 100% sensitivity, 85% accuracy and 100% precision outperforming other in vitro approaches. 15% intra-study CV and < 20% inter-study CV were observed for LDH, urea and albumin quality control metrics (N = 360).
Improve in vitro to in vivo translation
Improved data translatability from the lab to the human is achieved through dose–response experiments measuring clinical markers, such as ALT/AST.
Here, four highly hepatotoxic compounds were detected as true positives with ALT elevations aligned to patient data which were not captured in preclinical animal models.
| Nefazodone | Diclofenac | Sitaxentan | Troglitazone |
|---|---|---|---|---|
| DILI rank | 8 | 8 | 8 | 8 |
| Label section | Box warning | Box warning | Withdrawn | Withdrawn |
| In vivo animal models | Rats – decrease ALT Dogs – no ALT data | Rats –minimal ALT Dogs – no damage | Rats – no ALT data, Dogs – decrease ALT | Rats – minimal ALT Dogs – no ALT data, |
| Clinical ALT elevations | Modest elevations | 1-2 x ULN in 15% | 3 x ULN in 3-5% | >3 x ULN in 2% |
| PhysioMimix ALT elevations | 2 x ULV | 2 x ULV | >2 x ULV | >3 x ULV |
A viable path for human-specific drug modalities
In this publication, Sewing et al., (2016), cite “A series of reports describe changes observed in rodent and non-rodent studies related to SSO (single-stranded oligonucleotide)-induced toxicity, but as the safest preclinical candidates progress into the clinic, the human relevance of the observed effects remained unclear so far.”
Human-relevant markers from the PhysioMimx DILI assay, including alanine aminotransferase (ALT) and CYP3A4 activity, were able to clearly show and predict human outcomes between ASO-1 (toxic) and ASO-2 (safe), providing further clarity where animal model and 2D hepatocyte toxicology data were conflicting.
Generate high content data for a complete DILI profile
Our sensitive DILI assay provides high-content data from every replicate allowing the exploration of mechanisms of DILI alongside the assessment of a range of cell health markers.
Explore our Liver-on-a-chip models
Recreate the 3D multi-cellular architecture of the liver using perfused scaffolds.
Achieve longer-term viability, enhanced functionality & high metabolic activity.
Access our DILI Service
Get instant access to the PhysioMimix DILI Assay via our CRO Service. Through a collaborative approach, our experts work with you to plan and execute your study.
Standard and bespoke projects are carried out by our dedicated team of scientists in our CRO facility providing you with actionable data within weeks.
Add PhysioMimix OOC in your lab
Harness the power of PhysioMimix OOC in your own lab with the purchase of a single- or multi-organ microphysiological system.
With a growing community of users and support from our experts, there has never been a better time to transition into 3D cell culture.
PhysioMimix DILI assay kit: Human 24
The all-in-one DILI assay kit contains everything you need to recreate our FDA-recognized human assay in your lab.
Rapidly bridge the knowledge gap between traditional in vitro and first-in-human studies without the need for model/assay development and validation. Compatible with all PhysioMimix OOC Systems.
Frequently asked questions
Note that the terms organ-on-a-chip (OOC) and microphysiological system (MPS) are used synonymously throughout.
Before running your PhysioMimix Drug-induced liver injury (DILI) assay, do you pre-qualify your primary hepatocyte and Kupffer cells? If so, what does a validation study look like?
Qualifying primary cells prior to running a PhysioMimix® Organ-on-a-chip assay is key to a successful experiment and should not be overlooked. Firstly, starting with primary hepatocyte cells, we can culture several different lots in our Multi-chip Liver plates to ensure good three-dimensional (3D) tissue formation, physiologically relevant tissue functionality, metabolic activity, and extended longevity. Once we have identified primary hepatocyte lots that perform well i.e., within our pass/fail criteria, we co-culture them with Kupffer cells, test their response to an inflammatory stimulus, and examine the general tissue health and function over an 8 to 14-day period.
For more detailed information about the cell validation process and its importance, please read this recent Blog: Mirror, mirror on the wall, which is the best cell of them all?
How many endpoint readouts can you gain from each sample/chip in a PhysioMimix® Drug-induced liver injury (DILI) assay?
Our common endpoints are cited above; however, this is by no means an exhaustive list of detectable endpoints for the assay. As we can extract and sample a large volume of media from each chip (up to 1 mL per chip from Multi-chip Liver-12 plates and up to 0.25 mL per chip in the Multi-chip Liver-48 plates), many additional endpoints can also be measured in parallel to deliver detailed mechanistic insights into a drug’s toxicity profile. For example, a Luminex assay panel may contain 20 or more analyte readouts. Clinical biomarkers, such as AST/ALT, can also be detected offering advantages over traditional in vitro cultures. Furthermore, the scaffold containing the liver microtissues can be removed for additional endpoint analysis, such as staining for microscopy, or extraction of protein/RNA/DNA for screening.
What is different about your PhysioMimix® Drug-induced liver injury (DILI) assay that enables clinical measurements such as ALT/AST to be measured?
The PhysioMimix DILI assay’s ability to detect clinical measurements, such as ALT/AST, relates to the relatively large microtissues that are cultured in our Multi-chip Liver plates. Other systems are limited by the small amount of cells/supernatant that is available for sampling from their assay. Our cell-to-media volume ratio is key to accessing a wide range of metabolites and biomarkers. The PhysioMimix Liver-12 plate Liver-on-a-Chip model comprises up to 6 x 105 primary human hepatocytes that are perfused by a large volume of media (up to 1.6 mL). This provides the sensitivity required for the reliable quantification of clinical markers that are notoriously challenging to measure in other in vitro models.
For more information about how the PhysioMimix DILI assay provides greater assay sensitivity and the benefits of this approach for de-risking DILI predictions, please read the following Blog: De-risking drug-induced liver injury through the predictive power of organ-on-a-chip.
What are the advantages of your PhysioMimix® Drug-induced liver injury (DILI) assay versus other 3D models such as spheroids?
Spheroids are limited to some assays as they only contain 1,000 to 3,000 cells. They are typically cultured under static cell culture, which leads to necrotic cores – cell death at the center due to a lack of nutrients and oxygen, after just a few days. Even when perfused, these models tend to apply a gravity-driven perfusion mechanism, so, cells at the center continue to suffer poor access to nutrients and oxygen compared to the cells on the outer edge of the structure. This limits their longevity. Spheroids also suffer from loss of function over time (de-differentiation) and display comparatively low metabolic activity. Our PhysioMimix OOC systems culture larger-scale 3D liver microtissues, which mimic the architecture of the liver sinusoid. We maintain microtissue viability, phenotype, and metabolic activity for over 30 days by perfusing individual microtissues via fluidic flow. Although most of our customers prefer to capture their DILI data over 8-day experiment periods the PhysioMimix DILI assay allows for longer-term assessments of toxicity, the detection of clinically-translatable biomarkers, and more complex biological interactions to be studied if required (Long et al., 2016).
For more information about how the PhysioMimix DILI assay provides greater assay sensitivity and the benefits of this approach for de-risking DILI predictions, please read the following Blog: De-risking drug-induced liver injury through the predictive power of organ-on-a-chip.
Can you investigate enhanced susceptibility to DILI using a fatty liver disease model?
Yes, you can. It usually takes 4 days to induce fatty liver diseases such as Non-alcoholic fatty liver disease (NAFLD), (also known as metabolic dysfunction-associated steatotic liver disease – MASLD), or non-alcohol steatohepatitis (NASH), (also known as Metabolic dysfunction-associated steatohepatitis – MASH) – in our tri-culture Liver-on-a-chip model. Inducing acute or chronic DILI takes a bit longer, usually around 14 days. Diseased 3D Liver microtissues can be cultured and treated with drugs or compounds of choice for 14 days or more.
To learn more about our fatty liver diseases and DILI model, download Application Note: Microphysiological System for Studying Fatty Liver Diseases and its Impact on Drug-Induced Liver Injury.
Can your PhysioMimix Drug-induced liver injury assay address immune-mediated DILI?
Co-culture models containing primary human hepatocytes (PHH) and human non-parenchymal cells (Kupffer cells) are used to assess innate immune-mediated toxicological responses as standard, increasing the assay sensitivity versus primary PHH cultures.
Peripheral immune cells can also be added into the media that perfuse the liver microtissues to assess adaptive immune responses. However, we do not have a fully validated SOP for this application yet. Contact us for more information, or to register your interest.
Have you tested any advanced drug modalities in your PhysioMimix® Drug-induced liver injury (DILI) assay?
Yes, the MPS model provides a viable alternative to animal models, where human translatability is known to be poor. New modalities, such as antibodies, RNA therapies and cell-base therapies, are difficult to test in animals as they are specific to humans, and animal models often lack the correct targets or pathways to produce useful results. Our human-relevant DILI model is well suited for testing the interaction of these new therapeutics with the liver.
For more information, please read our Application Note: Human liver microphysiological system for predicting the drug-induced liver toxicity of differing drug modalities.
Have you compared the results of your PhysioMimix® Drug-induced liver injury (DILI) assay to in vivo animal studies?
We have tested our MPS models against in vivo models to reiterate that some interspecies differences can lead to discrepancies between the predictions observed in out humanized assay compared to in vivo animal studies. Look out for our preclinical animal MPS comparative datasets in 2024.
What is the sensitivity accuracy and specificity of your PhysioMimix® Drug-induced liver injury (DILI) assay?
Using reference compounds (IQ MPS Consortium DILI validation set) our human PhysioMimix DILI Assay (using the Multi-chip Liver-12 plate) delivers 100% sensitivity, 85%, accuracy and 100% precision. As well as giving “yes/no” predictions for DILI positive and negative compounds, the model gives multi-parametric detail to the nature and extent of DILI severity. Clinical markers such as ALT can be directly compared to clinical ULN (upper limit of normal) metrics. Together, this demonstrates the power of the PhysioMimix DILI assay to predict and quantify DILI risks.
What is the throughput of your PhysioMimix® Drug-induced liver injury (DILI) assay?
Up to six Multi-chip Liver-12 plates can be run simultaneously by one PhysioMimix OOC Controller unit, providing 72 samples per run for investigative studies that require deep insights into mechanism of action.
Alternatively, we have recently developed a miniaturized Liver-48 plate for use in a higher throughput setting such as lead optimization. Currently, three Liver-48 plates can be simultaneously run using one PhysioMimix Single-organ HT controller unit, providing 144 samples/run capacity.
What is the physiologically relevant flow rate for liver? Should the liver experience any meaningful shear?
In the liver, hepatocytes experience very low (near zero) shear stress as they are separated from the blood by the fenestrated (i.e. has holes) endothelium. However, as the endothelium is fenestrated, there is substantial mass transport, and this is influenced by the flow rate in the sinusoid. Our PhysioMimix OOC Systems provide sufficient flow to ensure good mass transport with low shear stresses of a similar order of magnitude to those observed in the liver sinusoid/capillary. To learn more about the flow in our system please read Domansky et al, 2010 and Ebrahimkhani et al, 2014.
Do liver-on-a-chip microtissues develop bile canaliculi?
We have stained the liver microtissue for markers that are known to be trafficked into the canaliculi space. We have also labelled it with antibodies for the expression of markers on the canaliculi. When looking at scanning electron microscopy (SEM) image of the actual liver-on-a-chip tissues, we observe lumens, with microvilli formation, developing for the bile. (Please refer to the image in the “Dynamic 3D microenvironment” section above).
How do you confirm the presence of different cell types in your organ-on-a-chip models?
When developing a co-culture model, confirming the presence of the required cells is critical. Depending on the organ that you are trying to recapitulate and its composite cell types, specific biomarkers can be used to confirm the presence of the different cell types.
In our duo-, and tri-culture liver models, Kupffer cells are incorporated to represent the liver’s innate immune system. Their presence is confirmed by measuring inflammation levels, via inflammation biomarkers (such as interleukins or cytokines) and compared to the control – primary human hepatocytes monocultures (PHH).
In our triculture model of Non-alcoholic steatohepatitis (NASH), stellate cells are added, and their presence is confirmed by comparing fibrosis levels to PHH monocultures in a fat media (Kostrzewski et al 2021). Transcriptomic analysis to detect the RNA expression of cell-specific markers provides an alternative or additional approach to confirm cell types in the culture.
How does the Liver-on-a-chip model perform compared to other in vitro alternatives?
Simpler in vitro systems successfully flag “yes/no” drug responses. These are perfectly complemented with the use of more advanced perfused Organ-on-a-chip (OOC) models to provide detailed data. To achieve data richness and high assay sensitivity, we require large cell culture volumes. In the Liver-12 plate, approximately half a million primary cells, perfused by large volumes of media (>1 mL), generate enough horsepower to reproduce phase 1 and 2 metabolism (particularly important because one of the reasons standard animal and in vitro models have proven to be poor predictors is due to their non-human metabolic profiles) and to increase assay sensitivity so more sophisticated clinical chemistry outputs of liver function, such as ALT/AST concentrations, can also be detected.
Samples taken over time in longitudinal studies provide biochemical assessments that build up a picture over time. This (relatively) large amount of tissue recovered at the end of the experiment can also be examined in additional proteomics or genomics studies, to investigate gene up- and down-regulation to generate a more complete mechanistic profile of drug action.
Hepatocyte performance in PhysioMimix OOC Systems is significantly greater compared to other standard approaches such as collagen-sandwich cultures and spheroids. CYP activity can be detected throughout the culture period, as can the expression of transporters.
For an independently generated comparison please see our peer reviewed co-publication with the US FDA
For more information read the following Blog : De-risking drug-induced liver injury (DILI) through the predictive power of organ-on-a-chip.
How do you account for donor-to-donor variability in Liver-on-a-chip models?
Accounting for door-to-donor variation has always been a challenging in in vitro experiments. There are several things to consider when donor-donor variability is considered:
- What is the adequate number of donors to include in a study, without drastically increasing the cost and timeline of the study?
- What genetic, gender, or ethnic background should we look for in the different donors?
- Can we access a donors that allow for diversity and also perform well under my experimental conditions?
When validating donors, we always try to find the right balance between the requirement for the different donors and the quality of donors. It is important to source cells from more than one supplier as access to the number of different donors greatly depends on the cell suppliers’ availability.
As previously mentioned, the number of donors that you evaluate within a study affects the cost of the study. So, the rule is simple, the more donors you incorporate, the more expensive the study will be. One way to minimize cost is to use pooled donors, which is a good compromise for rapid assessments. However, in our experience, pre-mixed pooled donors purchased off-the-shelf generate poor quality microtissues in OOC culture.
If you want to run a study using pooled donors, we recommend identifying three to five donors that have passed the pre-validation tests individually (to test for liver microtissue formation and function) before pooling them together (Tsamandouras et al, 2017).
For more information, read our Blog: A guide to pre-validating primary cells for use in organ-on-a-chip assays.
For more info about 3D validating cells, or to find out how to circumvent this step you can source 3D validated cells from us directly.
As practical as pooled donors may be, they do have limitations. For example, one donor may be more represented than the others in the microtissue, as we cannot control which donor cells attach best to form the microtissues in the OOC plates.
Using a higher throughput OOC system can help reduce the financial and resource burden for such experiments, as more conditions can be combined and studied on each Multi-chip Liver plate. For example, our PhysioMimix Single-organ HT System and its Liver-48 plate enables four times (n= 16 conditions in triplicate) the experimental capacity of our standard PhysioMimix Liver-12 plate.
This poster from the US FDA: Characterization of the effect of primary human hepatocyte (PHH) lots on function in CN Bio Innovations Liver-Chip using acetaminophen, provides some useful insights into the effect of donor-to-donor variability in OOC assays.
If you do not find the answer to your question listed, please contact us