It’s commonly acknowledged that the current drug discovery process is inefficient with large numbers of drugs failing in the clinic. Almost a third fail due to undetected toxicity issues. Countless more potential drugs fail due to misclassification by animal models.
Discovering toxicity late in the process is costly for drug developers. Expensive clinical trials must be halted for further investigation of the mechanism of toxicity to understand causality. These late-stage failures result in significant lost sunk costs, missed return on investment and potential reputational damage. Similarly, misclassification of drugs and their removal from development due to unsuitable animal models results in potential revenue loss.
The problem is exacerbated by newer drug modalities, with human-specific mechanisms of action. Here, inter-species pharmacological differences can make the use of animals less suitable for their development.
Failure to modernize drug testing workflows will result in more of the same, with no reduction in attrition rates and the same element of risk within the process.
The industry needs to rethink its approach to find effective ways of addressing these problems. More human-specific tests that identify problematic drugs and their mechanisms of action during earlier phases of the process are required.
Physiologically relevant organ-on-a-chip (OOC) models, or microphysiological systems (MPS) as they are alternatively known, provide a unique approach to understand if exposure to an acute, or repeated dose, of an experimental drug is likely to cause toxicity to humans. They also help explain how the drug causes toxicity by revealing the underlying mechanisms via a plethora of high-content endpoint measurements, including clinically relevant biomarkers, delivering greater human translatability compared to traditional in vitro approaches. This enables better-informed decisions about which candidates to take forward into in vivo animal, or human studies and which to halt, or re-engineer for a more cost-effective process.
Here we discuss why it is so important to understand the mechanism behind a toxic effect, how OOC can support more insightful decision-making within safety toxicology workflows, and the benefits of acting quickly.
We need a deeper understanding of the mechanism of toxicity
Current drug discovery and development workflows rely on in vitro (cell line-based) assays that lack physiological complexity, in vivo (animal) models that lack human relevance, plus computational modeling and simulations that are only as good as the data that feeds into them! Ninety per cent of drugs entering into clinical trials that were developed using this approach fail (Sun et al., 2022). Roughly 30% are due to safety concerns and approximately 18% (Walker et al., 2020), are specifically due to drug-induced liver injury (DILI). Countless more potentially good drugs do not make it to the clinic because of concerns flagged during in vivo animal studies that may not have translated into human risk. This suggests that potential blockbusters may have been missed as a result.
The reality is exemplified by the fact that many current mainstay drugs would not have made it through today’s rigorous testing procedures ( Van Norman in 2019). We believe that OOCs provide more clarity to fail early, prevent unnecessary attrition and be better prepared for the clinic by preempting issues with mitigation strategies.
The process of discovering and developing drugs is not just inefficient but also costly. It is estimated to cost $2.3BN/drug to take a drug to market. In 2022, Pharma ROI dropped to an all-time low of 1.2%, partially due to the COVID pandemic but also due to longer cycle times, increasing costs to develop drugs and declining returns on drug sales (Deloitte report).
Additionally, the types of drugs in discovery and development are changing. We are observing an upward trend in the development of new advanced therapies, or ‘new drug modalities’ to address the limitations of traditional small molecules. These drugs target human-specific targets and pathways for which animal use is less suited due to physiological/ pharmacological differences. Figure 1 below shows the 10% increase in new modality drugs reaching the US market over the last 4 years (mustard, purple and salmon). This raises concerns as to whether the chosen species will accurately reflect human nuances.
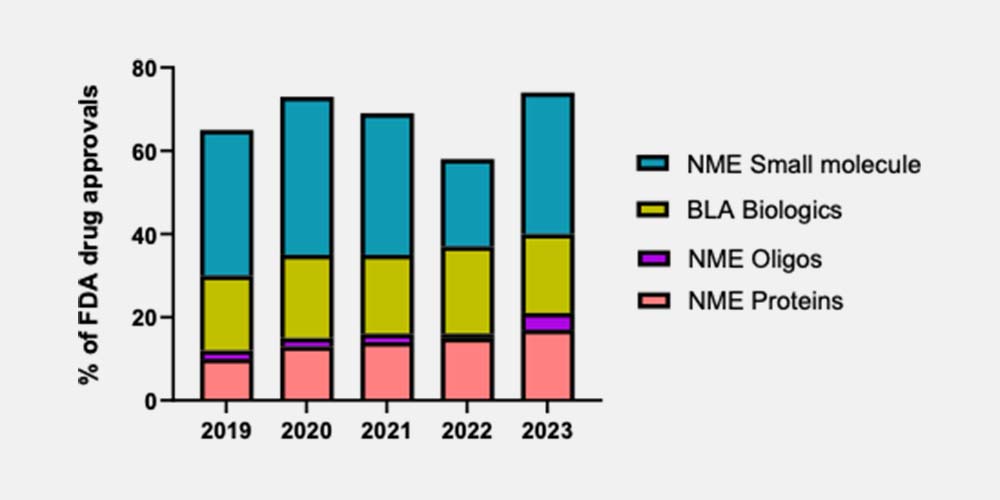
Fig. 1 FDA Drug approvals by drug modality
One mechanism of toxicity that is not well modeled by traditional preclinical approaches is the activation of the human immune system. Many adverse effects from newer human drug modalities arise because of a triggered immune response. Small molecules can also exert this effect. Capturing this adverse event is challenging in current models, as there are large species differences in immunity, whilst 2D models lack the complexity required. Here, OOC offers a unique path forward by providing the ability to incorporate various aspects of the immune system.
Furthermore, there are ethical considerations surrounding patient and animal safeguarding before in vivo studies to consider. A greater understanding of a drug’s toxicity profile during the discovery phase aids selection of the best animal species, thus aiding the refinement of preclinical assay design. Adopting animal OOC alongside human OOC can help design more effective and focused preclinical studies.
It is crucial to have a deeper understanding of a drug’s potential mechanism of toxicity to improve and modernize the drug development process. By identifying potential issues early, exploratory investigations to understand the cause can facilitate the recovery of good drugs by engineering out flaws or ceasing programs before they get expensive.
Unlocking mechanisms of toxicity using Organ-on-a-chip
When developing drugs, the liver is a key organ. As well as being central to the metabolism of many molecules, it also represents one of the primary tissues affected by drug-induced injuries (DILI). There are three main types of DILI: intrinsic, indirect and idiosyncratic. In the main, intrinsic DILI risk can be weeded out using the standard battery of in vitro tests. However, the latter two types require advanced assays that are more human-relevant, long-lived, highly functional, and metabolically active.
The PhysioMimix® Core MPS is particularly well-equipped for the job. Firstly, it doesn’t use PDMS in the consumable plates to minimize the non-specific absorption of drugs, or secreted cellular biomarkers from the cell culture media for more accurate predictions. Our PhysioMimix Liver-on-a-chip combines primary human hepatocyte (PHH) and Kupffer cells (KCs) to represent the organ’s innate immune system, which form microtissues within a perfused 3D collagen-coated scaffold. The model is used in the PhysioMimix DILI assay where hepatocytes express highly functional CYPs that facilitate the production of toxic phase I and II metabolites, human-specific transporters to identify transporter-mediated toxicity and bile acid machinery to detect cholestatic injury. The assay provides large amounts of recoverable material (media and tissue) for in-depth analysis to understand biotransformation and mechanisms of human toxicity. Glutathione (GSH) can be measured to highlight reactive metabolite toxicity/mitochondrial perturbation, in addition to other clinically relevant endpoints such as Albumin, Urea, ATP, ALT/AST, and miR122. The cell number and medium volume are optimized to allow RNAseq and -omics analysis from a single well to derive even more endpoints from each replicate. Plus, scaffolds are recoverable and amenable to confocal imaging for visual confirmation of results using immunofluorescent labeling.

The assay delivers exceptional performance as exemplified in a prior study using 13 reference compounds from the IQ MPS Consortium DILI validation set which delivered 100% sensitivity, 85% accuracy, and 100% precision using our Multi-chip Liver-12 consumable plate. Read more here in our Application Note which outlines the study in more detail. In summary, by measuring 6 different endpoint biomarkers, including clinical biomarkers, the model produced a “signature of hepatotoxicity” for each test article to help identify compounds with different levels of DILI concern. Due to its enhanced sensitivity, the model identified hepatotoxicants (pioglitazone, dexamethasone, levofloxacin) that were not detected using 2D and some 3D in vitro models. Furthermore, its ability to predict the toxicity of newer drug modality antisense oligonucleotides (ASOs) was demonstrated.
The approach has also been recognized by the U.S. FDA CDER (Centre for Drug Evaluation and Research) group, who cited superior performance versus standard approaches in the first publication between OOC provider and regulator. Several additional publications are available including Rowe et al., 2019, which explores the model’s use for identifying reactive metabolite-forming and mitochondria-perturbing hepatotoxins, Gallagher et al., 2023 who sought to identify significantly changing biomarkers and pathways in response to nerve agent exposure and Sakar et al in 2017, who performed an integrated assessment of diclofenac biotransformation, pharmacokinetics and omics-based toxicity in a 3D human liver-immunocompetent co-culture system.
Rethinking the drug discovery process
Recent technological advances enable OOC assays to be utilized earlier than ever before within lead optimization, enabling more insightful go/no go or reengineering decisions to be made at the tipping point between drug discovery and development. 90% of the costs of taking a drug to market are incurred from the lead optimization stage onwards (Sun et al., 2022). Adoption of OOC here offers significant financial benefits. By justifying the progression of only the most promising drugs into in vivo studies, the PhysioMimix Liver-48 DILI assay can reduce unnecessary animal use, potentially prevent the misclassification of drugs as toxic due to inter-species differences and facilitate the more confident progression of those with clean profiles into the latter more costly stages of the process.
CN Bio is working on data to demonstrate the PhysioMimix DILI assay’s ability to elucidate additional mechanistic pathways. A fully immunocompetent model to further increase assay sensitivity by including the adaptive element of the liver’s immune system (e.g. circulating peripheral blood cells seeded into the system’s fluidic flow) is in development to address this unmet need. The feasibility of a fully immunocompetent model has already been demonstrated by our customers and presented at past Society of Toxicology Annual meetings. We are also working on preclinical animal OOC models/ assays that are coming soon to support the further refinement of preclinical in vivo assay design.
OOC’s place alongside traditional methods
All preclinical tests are models, and all models have limitations. For this reason, we believe that OOC should be used in a complementary manner. OOCs provide more physiologically relevant and deeper mechanistic insights compared to traditional in vitro assays to confirm and understand the reasons behind potential liabilities in humans. However, they operate in isolation e.g. as a single-organ (liver-on-a-chip), or as interconnected multi-organ systems. A whole living organism is therefore the only option for exploring systemic toxicity.
Furthermore, until regulatory agencies accept data derived exclusively from OOC models as an alternative, animal use will continue across the board. However, the complementary use of OOC to refine in vivo study design and reduce unnecessary animal use offers significant workflow advantages over the current status quo.
The importance of acting quickly
The current cost and complexity of drug discovery is not sustainable in the long term. Change is required to reduce drug attrition rates and support a better return on drug discovery and development costs.
Traditional in vitro assays are too simplistic and in vivo animal models are not human, but OOC bridges the human relevance gap. OOC technology is rapidly becoming a lab essential (Authier et al., 2021 & Pognan et al., 2023) that is integral to safety toxicology workflows because it enables better-informed decisions about which drug candidates to select so that only the most promising candidates pass through. For newer human-specific drug modalities, they offer a viable alternative to be better prepared for first-in-human studies.
Regulatory submissions using OOC data have now been made. The PhysioMimix NASH assay played a crucial role in confirming the efficacy of INI-822 from Inipharm in its recent regulatory submission for non-alcoholic steatohepatitis (NASH). More will undoubtedly follow. Companies need to act now to remain ahead of the curve.
To facilitate the adoption of OOC, we have designed the PhysioMimix DILI Assay kit: Human 24. The kit contains everything required to recreate our FDA-recognized assay in your laboratory. Combined with comprehensive support from our experts, we can help you begin the process of future-proofing your safety toxicology workflows, starting with DILI research to improve your chance of clinical success in the future. So, what are you waiting for?




