How mimicking blood flow makes Organ-on-a-chip technology even more life-like
With every beat of your heart, blood rushes through your body. Some floods your arteries traveling from the lungs back to the heart. Some squeezes almost single-file, one frisbee-shaped cell at a time, through the capillaries in the thinnest layers of your eyelid.
As blood circulates, it exerts force on everything it touches – the force is felt in every organ of the body. But what happens when scientists want to recreate an organ outside of the body using technology such as Organ-on-a-Chip (OOC)? Without the forces exerted on tissues by blood flow, these lab-grown organs and tissues may not develop properly, resulting in organs that don’t function the same way as or deliver experimental data representative of their human-body counterparts.
So, when it comes to the future success of Organ-on-a-Chip technology, alternatively known as known as a Microphysiological System (MPS), how crucial is the incorporation of fluid flow to mimic the circulatory system?
What is flow?
Simply put, flow is the movement of fluid through a vessel. As a fluid moves, which in the case of blood could be through a wide artery or an infinitesimally narrow capillary, it pushes against the walls of its vessel exerting shear forces on the vessel walls. Research1 has shown that the shear stress exerted by blood flow is integral for proper tissue and organ development and function.
To understand the different shear stresses that blood flow exerts on tissues, bioengineers and scientists need to take into account both the viscosity of blood as a fluid, the size of different blood vessels through which it flows and the profile of the flow, is it smooth or pulsatile. All of this helps them to elucidate fundamental processes, critical to physiology such as the rate at which nutrients can be transported from the blood into the tissue cells and how well waste products are removed. All these calculations need to be integrated into the design of individual OOC models.
How do Organ-on-a-chip platforms regulate flow?
Taking a cue from the human heart, OOC bioengineers use pumps to push fluid through their systems, recreating the natural forces of blood rushing through an organ. But not every organ in the body experiences the same force of blood flow. For example, major blood vessels such as arteries experience high forces which change many times a second, while the narrow capillaries within tissues see a much steadier, gentler set of flows and forces. So dependent on the organ or tissue that is being emulated, the OOC system should provide flow that is strong and pulsatile, or slow and steady. Many pumps used in OOC systems produce pulsatile flow, which is great if you want to make an artery. The trick if you want to model other organs, such as the liver which has a much steadier flow, is to damp out those pulses.
How does incorporating flow into Organ-on-a-chip technology improve upon in vitro 2D culture?
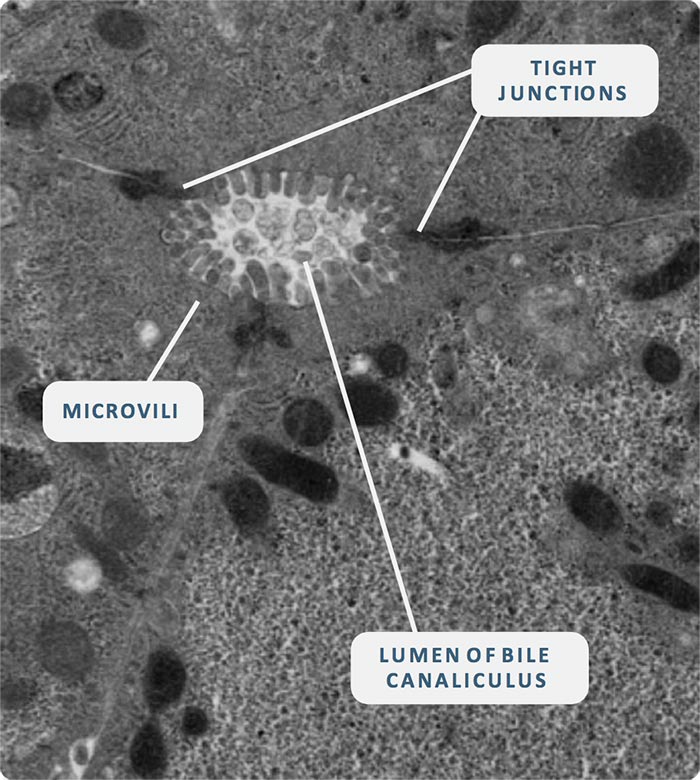
Flowing from organ to organ
In the human body, blood doesn’t just flow to and from one organ at a time. It moves from organ to organ, carrying nutrients, drugs, signaling proteins and metabolites from one part of the body to be used by the next.
An example is the connection between the gut and the liver. At CN Bio, we have recently developed a Multi-organ Gut-Liver model, which mimics blood flow between the organs. This is ideal for testing the effects of first pass metabolism, an important consideration for oral medicines, where significant amounts of drug can be changed to inactive form before they ever reach the systemic circulation and the desired target organ. This effect used to be measured with animal models and in clinical trials, now it can be explored in the lab.
Now we have systems which allow organs and tissues to talk to one and other, as they do in the body the potential is huge, enabling scientists to develop a deeper understanding of how drugs are absorbed, interact with, and affect multiple organs at the same time. For example, connecting liver tissue with another organ opens the opportunity to study reactive metabolite-driven toxicity or to simultaneously evaluate drug absorption and metabolism. Adding in circulating immune cells would enable investigators to look at how inflammation can mediate toxicity.
However, whilst the realms of possibility seem almost endless, so are the challenges! For example, let us start with emulating blood, i.e. the task of media selection. We all know that use of a tissue-specific media is key to culturing healthy and phenotypically functioning organs in vitro but what happens when researchers want to link organs together? One media type may facilitate the growth of one organ but be detrimental to another. To solve this we mine physiological data, sorting through effects and carefully adding or excluding different media constituents. Next skillful bioengineering is required to establish a physiologically-relevant flow rate and cardiac output both within each organ and between the different organs in the model.
These are the challenges that CN Bio tackles head-on, using our unique expertise and experience to develop Multi-organ models that can improve the accuracy of drug discovery and development. To date, animal models, with all their well-known limitations or computer simulations, were the only alternatives. Now organs and tissues composed of human cells can talk to each other in the lab for many weeks, unlocking the answers to key questions for drug discovery scientists.
By incorporating flow into organs, bioengineers at CN Bio have enabled OOC technology to more closely recapitulate how organs function in the human body, and by using our control of flow to connect multiple organs together, CN Bio enables scientists and clinicians to form a more complete picture of potential drugs’ effects. Together, by delivering results in the lab that translate into human outcomes, our advancements are rapidly closing the gap between 2D in vitro cell culture and clinical research.
With the rhythm of blood pulsing through them, our organs-on-a-chip come to life.
More information about our PhysioMimix® OOC Microphysiological System can be found here
Research article: Fluid shear stress threshold regulates angiogenic sprouting. Galie et al. (2014)
The Rhythm of Life
Author: Dr Stephanie De Marco


