Resource > Articles >
Next Generation Drug Discovery: Bridging the Gap with Organ-on-a-Chip Technology
Organ-on-a-chip: Bridging the in vitro to in vivo gap
Filed under: MASLD/MASH
In today’s drug development landscape, there is a critical need for more human-relevant preclinical testing models. Traditional in vitro assays and in vivo animal models often fall short in predicting human outcomes, especially as advanced drug modalities with human-specific actions become more prevalent. Organ-on-a-Chip (OOC) technology, a groundbreaking solution designed to replicate human physiology and function in vitro, offers a promising path forward by bridging the in vitro to in vivo gap.
A key player among New Approach Methodologies (NAMs), OOC aims to overcome the limitations of traditional models by accurately replicating human organ microarchitecture, microenvironment, and function. By culturing primary human cells in a perfused environment, OOC can recreate both healthy and diseased human organ mimics in the lab. This innovation is particularly valuable for testing advanced drug modalities where human-specific targets and pathways are crucial.
OOC technology enables the visualization of cell and tissue behaviour over time, providing insights that traditional in vitro models cannot. It enhances our understanding of inflammatory diseases by replicating the complex immune responses seen in humans.
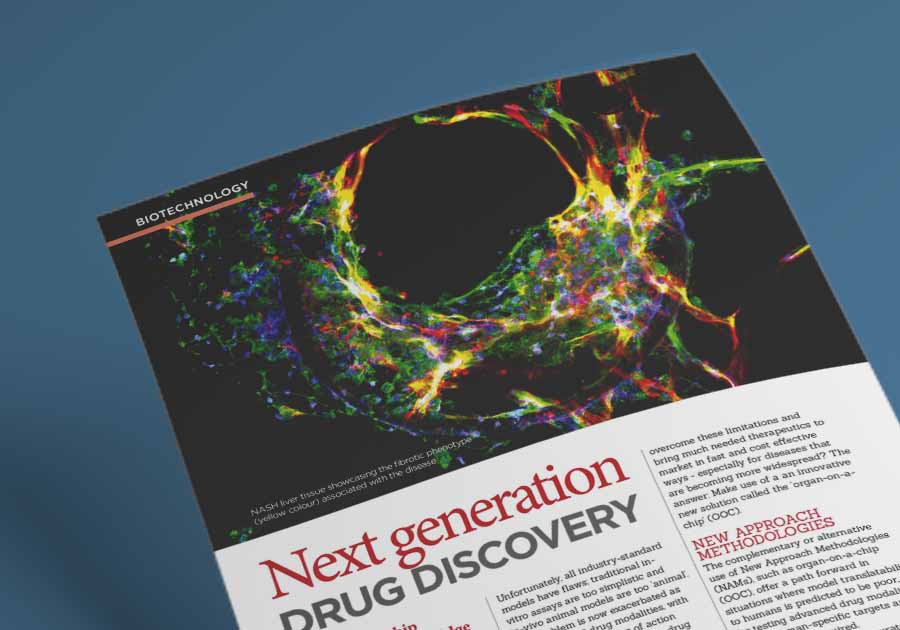
Non-alcoholic steatohepatitis (NASH), also known as metabolic dysfunction-associated steatohepatitis (MASH), is a severe form of chronic liver disease that has been notoriously difficult to treat due to the inadequacies of traditional in vivo models. CN Bio’s PhysioMimix® NASH-in-a-box assay uses primary human hepatocytes, stellates, and Kupffer cells to form 3D microtissues, capturing key stages of disease progression. This NASH OOC model supports drug development by uncovering precise mechanistic effects of drug efficacy, offering a more accurate prediction of human responses.
In December 2023, CN Bio’s Contract Research Service (CRS) supported the characterization of a drug candidate, leading to the initiation of a Phase 1 clinical trial for Inipharm’s INI-822. This milestone marks the first instance of OOC data supporting the clinical progression of a drug for a complex metabolic liver disease.
While animal models provide a comprehensive view of an organism’s complexity, OOC technology demonstrates how disease mechanisms and drug effects differ in humans. The combination of both models can offer broader preclinical insights, enhancing the drug development process.
This article was originally published in Eurolab’s June 2024 edition: Next-generation Drug Discovery. Read the full article here.


