Event > Conference >
EUROTOX 2023
The 57th Congress of the European Toxicologists and European Societies of Toxicology will take place this year under the theme “Toxicology – multidisciplinary science leading to safer and sustainable life.”
Will you be there too?

Head to our exhibition stand – #30
Our dedicated team will be available to answer questions and share over a decade of organ-on-a-chip (OOC) expertise with you at EUROTOX 2023!
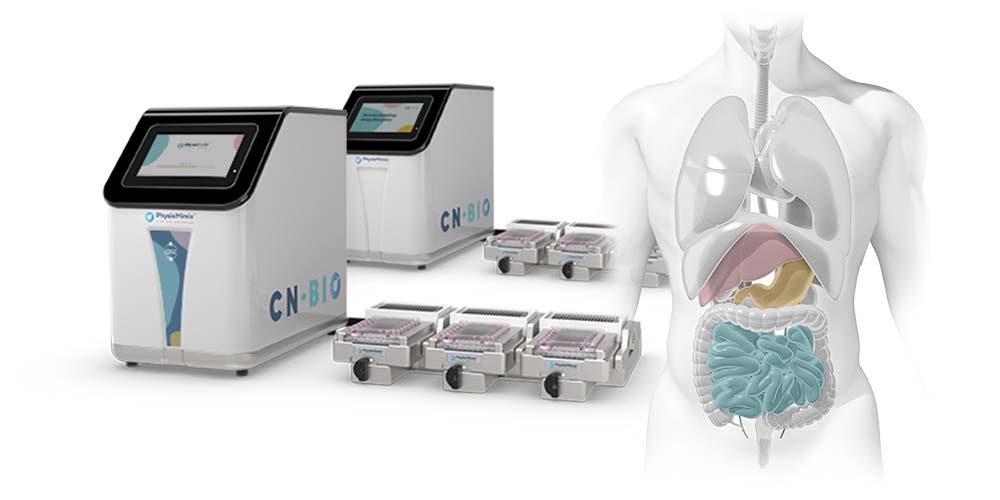
We look forward to introducing you to our PhysioMimix® suite of hardware, consumables, assay protocols and contract research services. Discover how our solutions deliver clinically relevant mechanistic data that enable you to design cost-efficient in vivo studies (using fewer animals) and improve your ability to predict clinical outcomes.
Plus, meet the new game-changing product in our PhysioMimix OOC range of microphysiological systems, the PhysioMimix Single-organ Higher Throughput (HT) System and associated Multi-chip Liver-48 plate.
A sensitive and robust human liver microphysiological system for assessing drug-induced liver injury
Drug-induced liver injury (DILI) remains the most common cause for acute liver failure in the USA and Europe and is a leading cause of attrition of compounds in drug development. As an alternative to classical 2D cell cultures, which have significant limitations in assessing DILI, we have developed a human liver microphysiological system (MPS) comprised of human primary liver hepatocyte parenchymal and non-parenchymal cells (NPCs), cultured in 3D microtissues on an engineered scaffold under perfusion up to two weeks. The methodology has been qualified with a broad set of thirteen severely and mildly hepatotoxic test articles aligning with the IQ Consortium MPS affiliate recommended list of small molecule drugs for predicting DILI.
Liver function following drug exposure was assessed by a broad spectrum of functional liver-specific endpoints on the cellular structures and culture medium (LDH, urea, CYP3A4, ATP), including clinically relevant biomarkers (e.g., albumin, ALT).
Robustness of the model was calculated for three main soluble biomarkers that are measured in the cell culture media at Day 4 to assess the liver microtissues quality control. Excellent intra-study coefficient of variance (CV) was calculated for LDH, urea and albumin (below 15%) and inter-study CV falls below 20% (N = 360 wells/liver microtissues) proving a sensitive and robust in vitro liver model for predicting DILI.
At a threshold of 50x Margin of Safety (MOS, ×Cmax) our liver MPS in vitro model showed superior sensitivity and specificity over classic 2D primary hepatocytes cultures and even some standard non-MPS 3D models in detecting DILI (with sensitivity 100%, accuracy 85%, and precision 100%). In addition, the liver MPS model correctly identified as True Positives all tested compounds known to give cholestatic hepatotoxicity (troglitazone, nefazodone, chlorpromazine and sitaxentan). More importantly, measured ALT levels in the cell culture media at 48 hours of exposure (expressed as fold increase to vehicle control) matched the severity grading in DILI set out by the Drug-Induced Liver Injury Network (DILIN) based on clinical data for all tested compounds, highlighting the clinical translatability of our liver MPS model.
Due to the human relevance and predictivity of the system if the liver MPS had been utilised in early drug development the hepatotoxicity of pharmaceutical compounds would have been identified at a much higher rate, helping to de-risk drug attrition in clinic. Subsequently, mechanistic insights of the toxicity may be investigated and a better alternative designed, saving cost in both drug development and human lives.
Date: Monday 11th September
Time: 9:30 am.
Poster Group/Number: Late breaking abstracts LP-35
Abstract: 899
Presenter: Dr Ovidiu Novac, Senior Scientist | CN Bio
Our Team at EUROTOX 2023

Adrian
A seasoned business developer with extensive commercial experience in the life science sector, Adrian Rea joined CN-Bio Innovations as the European Director of Sales in June 2022. He brings valuable experience in the 3D cell culture market from his most recent role at InSphero, where he was responsible for developing business opportunities in European and Asian markets. He was previously Sales Director at Enzo Life Sciences, where he managed the restructuring of the global distributor sales channels and expanding the European sales operations. Adrian graduated from the University of Glasgow and received his PhD in pharmacology from Glasgow Caledonian University.

Atefeh
Dr Atefeh Mobasseri, Field Application Scientist at CN Bio, has an extensive background in tissue engineering and regenerative medicine. Before joining CN Bio, she gained a PhD in Biomedical materials from the University of Manchester and carried out postdoctoral roles at the University of Manchester and Kings College London investigating the interaction, and effect, of 3D scaffolds on cellular behaviour. Since joining CN Bio, she has been supporting their European customers in using CN Bio’s range of PhysioMimixTM systems to generate high content, human-relevant, data.

Emily
Dr Emily Richardson is a Lead Scientist in the R&D team at CN Bio. She joined the team in 2020 as a Senior Scientist and lead the development of the PhysioMimix lung and lung-liver MPS models. Emily is highly experienced in the application of complex cell biology to drug discovery, having previously worked in cellular therapeutics in an industry setting and as a trained biochemist with specialty in molecular biology. She completed her PhD at the University of Leicester, using 3D cell culture to determine molecular mechanisms driving highly metastatic lung cancers. She now leads R&D projects within the CN Bio team revolving around toxicology in the liver and the lung MPS, as well as driving collaborative projects with various academic, industry and regulatory partners.

Ovi
Dr Ovidiu Novac is a Senior Scientist at CN Bio and a knowledgeable organ-on-a-chip (OOC) expert. Ovi performed as lead biological scientist in the NASH-in-a-Box project, is a knowledgeable organ-on-a-chip (OOC) expert, and has experience in assessing drug induced liver injury in vitro. Ovi has a background in the developmental stage of pharmaceutical and dermatological formulations and their performance assessment on human skin tissue ex vivo (topical and transdermal), and for strategic planning for further evaluation. During time spent in the pharma industry he contributed to delivering high quality results used for marketing purposes and in regulatory submissions. Ovi holds a BSc in biomaterials and prosthetic technologies and a PhD in chemistry and has been involved in research on controlled drug delivery systems since 2006.

