Resource > Application notes >
Enhance IVIVE with cross-species Liver MPS DILI assays
Unlock our cross species Liver microphysiological system (MPS) application note “Analysis of the PhysioMimix cross-species DILI assay to mimic in vivo predictions of DILI risk
Filed under: DILI and Safety toxicology

Video content if present
Discover how PhysioMimix® Core delivers Liver MPS DILI assays that facilitate in vitro hepatotoxicity testing across human, rat, and dog models and enhance your ability to predict Drug-induced liver injury risk before the clinic.
What you’ll get
A practical, data‑rich summary of how PhysioMimix human, rat and dog hepatocyte microtissues are cultured, qualified, and used to predict in vivo safety profiles—complete with assay timelines, endpoints (LDH, ALT, urea, albumin), and cross‑species comparisons against established DILI ranks.
5 key cross-species DILI assay takeaways
- Viable and functional human and animal Liver MPS up to 14 days
- Predictions that catch bile‑acid and mitochondrial liabilities
- Unlock a better mechanistic understanding of interspecies differences to reduce misclassification
- Enhanced confidence in MPS to refine species selection, reduce unnecessary animal use and predict human outcomes
- A robust and fast method of reviewing conflicting animal study data to inform which best aligns with equivalent human data
Example data
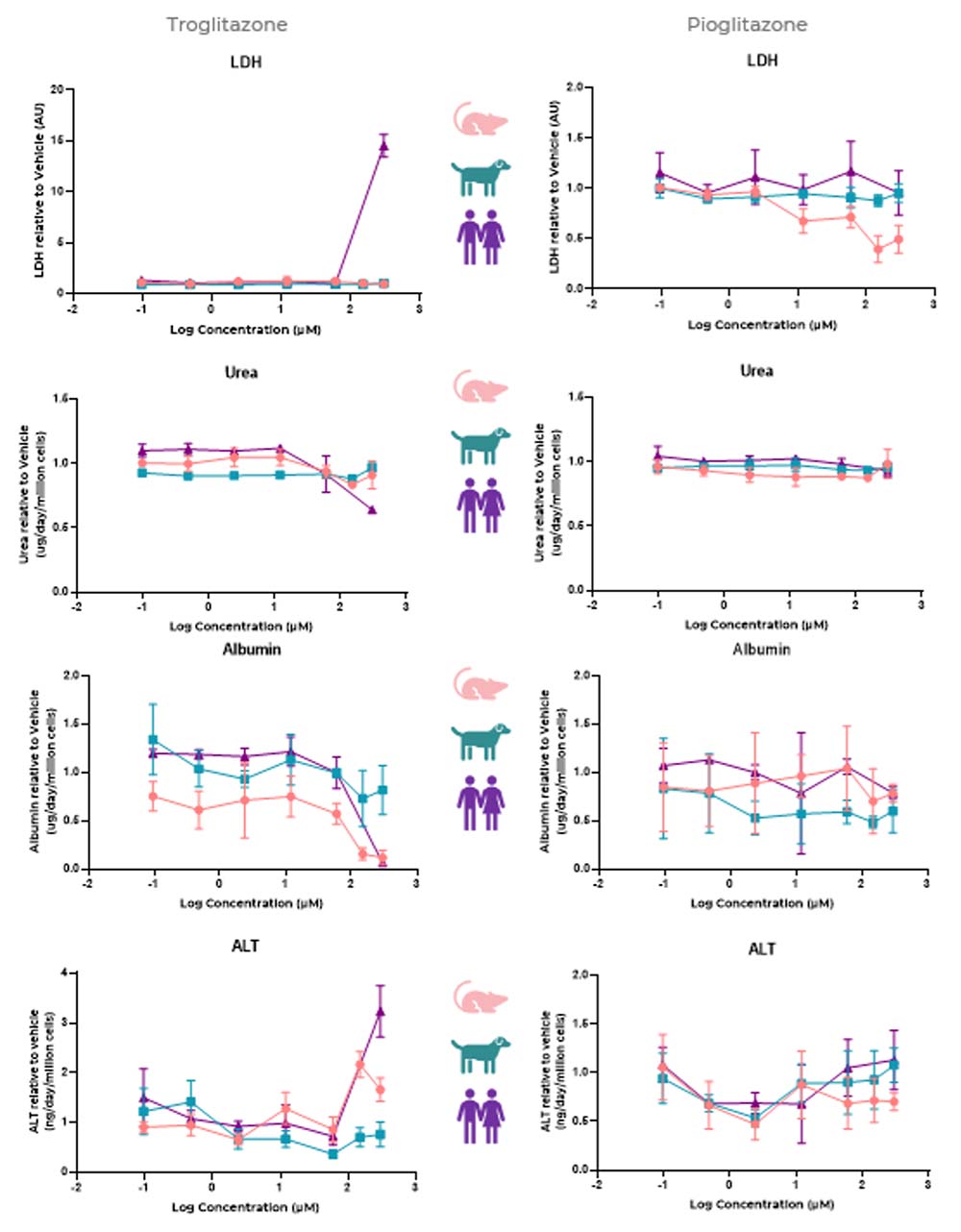
Rat MPS predicts the toxicity of troglitazone through reduction of albumin and increased ALT
Liver MPS-derived DILI profiles for troglitazone (high-DILI-concern, pink) and pioglitazone (low-DILI-concern, blue) using primary rat (left) and dog (right) hepatocytes with viability (LDH, ALT) and functionality (urea, albumin) endpoints. Liver microtissues in the MPS were exposed to troglitazone and pioglitazone for 96 hours. Endpoint measurements were all derived from the same liver MPS culture. Data shown are mean ± SD, N=3, and all from 48-hour samples.
Why now?
With regulators encouraging broader use of New Approach Methodologies (NAMs), our Liver MPS and DILI assays offer actionable, human and preclinical species relevant insight to complement and better inform animal studies – without waiting for late‑stage outcomes.
About the technology
The PhysioMimix® Core and Multi-chip Liver 12+ plate generates 3D liver microtissues with controlled perfusion, enabling high content mechanistic readouts from a single culture. Our technology aligns with a broader industry transition toward NAMs and improved translational relevance.
For more information about how to rapidly access our cross-species DILI assays, view our DILI in vitro Contract Research Services page, or Contact us


