Today, 11th November 2025, a much-anticipated press release from the UK government’s Science Minister Lord Vallance announced its plans to phase out animal testing faster, where reliable and effective alternative methods (such as organ-on-a-chip (OOC) and other New Approach Methodologies (NAMs) can replace them by offering the same level of safety for human exposure.
The proposed roadmap:
The UK’s plans to phase out animal testing set out a road map of commitments backed by £75M in funding, £60M will set up a new hub collating data, technology and expertise to encourage collaboration between researchers, and a separate new UK Centre for the Validation of Alternative Methods (UKCVAM) to streamline the path to regulatory approval for new alternatives. In addition, an extra £15.9M has been put forward by the Medical Research Council (MRC), Innovate UK and the Wellcome Trust to advance new in vitro models of the liver, brain, cancer, pain and blood vessels for use as more human-relevant alternatives to animals within five UK-based teams.
The roadmap cites:
- By 2030, reduce pharmacokinetic studies using dogs and non-human primates
- By 2027, end tests evaluating the strength of Botox on mice and to use only DNA-based lab methods for adventitious agent testing (virus, bacterial medicine contaminants)
- End of 2026, end regulatory testing on animals to assess skin and eye irritation and skin sensitization of new treatments
Other commitments from 2026 include:
- providing training in alternative methods for early-career researchers
- publishing research priorities for alternative methods every two years
- strengthening the commitment of research funds and the visibility of alternative methods
- positioning the UK as a global alternative methods regulation leader
A full overview of the Policy Paper “Replacing animals in science: A strategy to support the development, validation and uptake of alternative methods.
The strategy sets out steps that the UK government will take over the next five years towards achieving its long-term vision of “a world where the use of animals in science is eliminated in all but exceptional circumstances.” The approach includes targeted priorities for tests that can transition quickly, medium-term developments, and areas where alternatives do not yet exist. While some animal research will remain necessary during the transition, the ultimate goal is a research ecosystem that relies on human-relevant, non-animal models. Progress will be monitored by a committee, chaired by Lord Vallance, government ministers and departments, regulators and funders.
Science Minister Lord Vallance said:
“Nobody in our country of animal lovers wants to see suffering and our plan will support work to end animal testing wherever possible and roll out alternatives as soon as it is safe and effective to do so.
This is a roadmap which will ensure government, businesses and animal welfare groups can work together to find alternatives to animal testing faster and more effectively”.
The UK’s alternative methods track record
The strategy builds on the UK’s strong track record in developing and adopting alternative methods, including our National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) – the first organization of its kind, which has delivered non-animal alternatives in areas like vaccines, backed by government funding. The UK is home to many Centres for excellence in the field including: Queen Mary University of London (QMUL) – Centre for Predictive In-Vitro Models (CPM), one of the largest and most advanced OOC facilities in the UK and leading Organ-on-a-chip technology provider, CN Bio.
What is CN Bio doing to support the UK plans to phase out animal testing?
As the UK accelerates its roadmap to phase out animal testing, CN Bio is delivering the human-relevant technologies that make this vision achievable—offering organ-on-a-chip solutions alongside scientific rigor.
Our new easy-to-adopt, adapt and scale PhysioMimix® Core System allows single-organ, multi-organ, and higher-throughput studies (up to 288 samples), making organ-on-chip technology practical for both the research (to allow creation of novel models) and drug development (by providing reliable and robust solutions) settings to accelerate roadmap goals.
PhysioMimix single- and multi-organ solutions deliver insights that traditional in vitro solutions and animal models cannot, from toxicity predictions to modeling complex diseases and profiling drug pharmacokinetics. Additionally, our solutions can be used in conjunction with advanced in silico computational modeling tools (AI, Machine learning) to enhance the in vitro to in vivo translatability of data.
Two recent examples of this are:
- A CN Bio publication (Abbas et al., 2025) showcasing how we integrated in silico tools and with Gut/Liver-on-a-chip to deliver more accurate pharmacokinetic (bioavailability) predictions that reduce our reliance on animal models, such as dog and non-human primates.
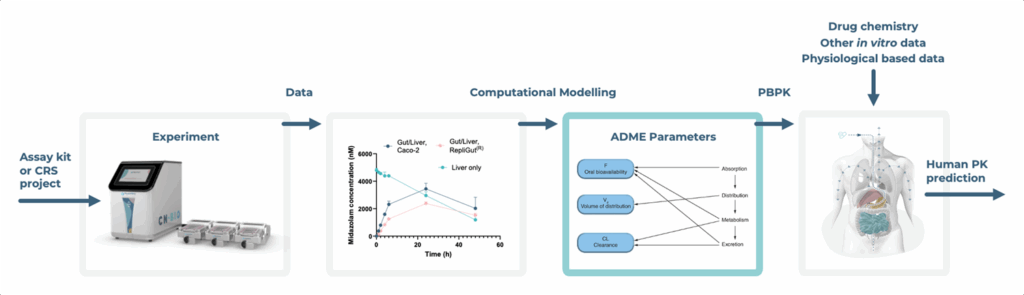
- Collaborators at Massachusetts Institute of Technology (MIT, Cadavid et al., 2025) developed a systems biology framework for the rational design of operational conditions for in vitro to in vivo translation of microphysiological systems (a synonymous term for OOC).
Our commitment to change
CN Bio’s CEO, Dr Paul Brooks, said,
“Today’s announcement marks a pivotal moment for science and innovation in the UK. At CN Bio, we’ve been at the forefront of developing organ-on-a-chip technologies for over a decade—pioneering human-relevant models that reduce reliance on animal testing while delivering more predictive insights for patient safety. This roadmap validates the approach we’ve championed and accelerates the transition to methods that are not only ethical but scientifically superior. We’re proud to lead this charge and stand ready to work with government and industry partners to turn ambition into action.”

At CN Bio, we are actively involved in numerous consortia groups, agencies, and networks to accelerate the phasing out of animal testing – which can be found on our about us page. Those relevant to today’s news include our close relationships with UK authorities and organizations that advise the government:
- NC3Rs: Active member of NC3Rs NAMs network. Strong relationships with key roadmap advisors. Awarded funding for CRACKIT challenge (SensOoChip)
- Innovate UK: Ongoing communication and previous grant funding for Lung-Liver COVID-19 project
- BSI/ISO: Member of BSI/ISO committee for discussions regarding MPS/OOC.
- QMUL: Industry Affiliate and member of the Centre for Predictive In Vitro Models (CPM), with a shared COaCT PhD student this year, focused on using PhysioMimix technology for oncology research.
- Animals in Science Committee: an advisory non-departmental public body, sponsored by the Home Office. Former CN Bio Lead Engineer, Dr. Dharaminder Singh, participating member.
- Animal Free Research and Centre for Human Specific Research: Active members
Why can you trust CN Bio’s PhysioMimix technology to deliver your roadmap changes?
CN Bio is an Organ-on-a-chip company with over a decade of experience. Our award winning PhysioMimix platform was commercialized from the “Legacy” prototype developed by Professor Linda Griffith’s pioneering laboratory at the Department of Biological Engineering, MIT. The technology has since evolved from 10+ years of know-how with single, multi-organ and higher-throughput options available within one PhysioMimix Core System.
PhysioMimix’s enhanced performance over traditional approaches, robustness and reliability are FDA-recognized. The technology has supported a successful regulatory filing for metabolic liver disease and has been widely adopted by 16 top major pharmaceutical companies.
Summary
There is no doubt that the regulatory landscape is shifting globally, with the US FDA and NIH already moving to prioritize non-animal methods; the UK has now followed to maintain its leadership in life sciences. The question is who’s next in line?
For researchers and drug developers alike, there’s no longer any uncertainty around the place of animal alternatives in your workflows. The field is growing momentum at a rapid rate, so, are you ready to get started?
We are committed to supporting global efforts to reduce animal use by enabling you to further our understanding of human physiology and disease and more efficiently develop novel therapeutics. Contact us to find out how we can support your journey with PhysioMimix Core.


